

Erik J. Sorensen
Contact:
Erik J. Sorensen
Arthur Allan Patchett Professor in Organic Chemistry, Professor of Chemistry
[email protected]
Frick Laboratory, 132
609-258-8135
Faculty Assistant:
Pattie Faranetta
[email protected]
Frick Laboratory, 228
609-258-5202
Research Focus
Our laboratory pursues ideas and chemical reactions that have the potential to advance the field of organic synthesis. The molecular architectures of physiologically active natural products inspire us to consider bold strategies for rapidly generating their intricate structures from relatively simple chemicals. We embrace risk-taking during the creative process of synthesis design and seek new ways for thinking about synthesis.
A student in our laboratory actively participates in the selection of the research problem and is integral to its design and execution.
Once we discuss the merits of a potential research problem and make a commitment to it, we turn our thoughts to how we might advance the broader field of synthesis through its pursuit. We begin by thinking about the problem from many different vantage points, and, in the wake of that process, we ask questions like:
(1) Does this molecular structure have an intrinsic potential for a structural self-formation from a much less intricate structure?; (2) Can we learn more about the scope of an emerging chemical reaction in the course of the project?; (3) Can we develop an innovative strategy in the course of the undertaking?; and (4) Can we contribute a new reagent or method for the betterment of the broader field of organic synthesis?
In the course of designing a research project, a student has an opportunity to think creatively, plan boldly, gain a deeper knowledge of the extant chemical literature, and place his/her thinking into the much broader context of the past achievements of organic chemistry.
In several, previously published projects from our laboratory, we set out to rapidly generate the molecular structures of biologically active natural products by channeling the reactivities of activated, polyunsaturated compounds. The keystone transformations for three of these prior efforts are shown in Figure 1. In each instance, the experimental effort identified reaction conditions under which the desired, multi-bond forming process could be achieved spontaneously.
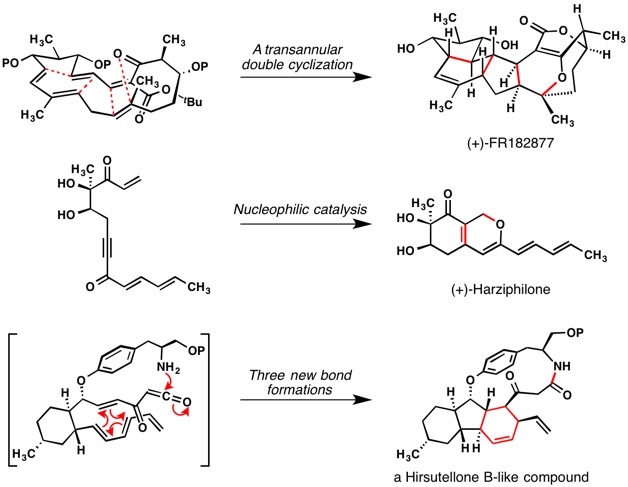
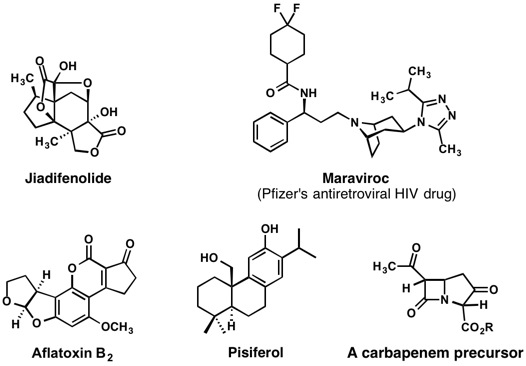
In addition to our interest in the concept of “architectural self-construction,” we are actively seeking creative opportunities for the expansive collection of methods that are capable of functionalizing unactivated carbon–hydrogen bonds. Our prior tendency to ask, “how might we leverage appropriately placed functional groups to do synthesis?” has given way to a bolder question of “how might we create multifunctional compounds through the execution of direct, site-selective functionalizations of unactivated C–H bonds?” As members of the NSF-sponsored Center for C–H Functionalization at Emory University, we are actively collaborating with several other research laboratories to demonstrate the impact that C–H functionalization methods can have on the planning and execution of syntheses of several target compounds. Some of our ongoing projects in this exciting area are shown in Figure 2.
Honors
Francis Clifford Phillips Lecturer, University of Pittsburgh (2016)
1st Gary Walters ‘67 Award for sustained commitment to the ideals of ‘education through athletics’ (2014)
1st Annual Carlos Montezuma Lecturer, University of Illinois at Urbana-Champaign (2013)
Honorary Member of the Graduating Class of 2011, Princeton University (2011)
Arthur C. Cope Scholar Award, American Chemical Society (2009)
Givaudan/Karrer Distinguished Visiting Professorship, University of Zürich (2007)
Bristol-Myers Squibb Unrestricted Grant in Synthetic Organic Chemistry (2004)
Pfizer Global Research Award for Excellence in Organic Chemistry (2002)
Camille Dreyfus Teacher-Scholar Award (2001)
Woodward Scholar, Harvard University (2001)
Beckman Young Investigator Award (2000)
Selected Publications
Siler, D. A.; Mighion, J. D.; Sorensen, E. J., “An Enantiospecific Synthesis of Jiadifenolide**.” Angewandte Chemie-International Edition 2014, 53 (21), 5332-5335.
Reber, K. P.; Tilley, S. D.; Carson, C. A.; Sorensen, E. J., “Toward a Synthesis of Hirsutellone B by the Concept of Double Cyclization.” Journal of Organic Chemistry 2013, 78 (19), 9584-9607.
Sorensen, E. J.; Siler, D. A.; Mighion, J. D., “Taking Risks in Complex Synthesis Design.” Strategies and Tactics in Organic Synthesis, 2013, Vol. 9, pp 249-273.
Kim, J.; Schneekloth, J. S., Jr.; Sorensen, E. J., “A chemical synthesis of 11-methoxy mitragynine pseudoindoxyl featuring the interrupted Ugi reaction.” Chemical Science 2012, 3 (9), 2849-2852.
Liu, J.; Lotesta, S. D.; Sorensen, E. J., “A concise synthesis of the molecular framework of pleuromutilin.” Chemical Communications 2011, 47 (5), 1500-1502.
Xie, H.; Sammis, G. M.; Flamme, E. M.; Kraml, C. M.; Sorensen, E. J., “The Catalytic Asymmetric Diels-Alder Reactions and Post-cycloaddition Reductive Transpositions of 1-Hydrazinodienes.” Chemistry-a European Journal 2011, 17 (40), 11131-11134.
Guerrero, C. A.; Sorensen, E. J., Concise, “Stereocontrolled Synthesis of the Citrinadin B Core Architecture.” Organic Letters 2011, 13 (19), 5164-5167.
Lotesta, S. D.; Liu, J.; Yates, E. V.; Krieger, I.; Sacchettini, J. C.; Freundlich, J. S.; Sorensen, E. J., “Expanding the pleuromutilin class of antibiotics by de novo chemical synthesis.” Chemical Science 2011, 2 (7), 1258-1261.
Freundlich, J. S.; Lalgondar, M.; Wei, J.-R.; Swanson, S.; Sorensen, E. J.; Rubin, E. J.; Sacchettini, J. C., “The Abyssomicin C family as in vitro inhibitors of Mycobacterium tuberculosis.” Tuberculosis 2010, 90 (5), 298-300.
Marsini, M. A.; Reider, P. J.; Sorensen, E. J., “A Concise and Convergent Synthesis of PA-824.” Journal of Organic Chemistry 2010, 75 (21), 7479-7482.
Chandler, B. D.; Roland, J. T.; Li, Y.; Sorensen, E. J., “Seebach’s Conjunctive Reagent Enables Double Cyclizations.” Organic Letters 2010, 12 (12), 2746-2749.
Spangler, J. E.; Carson, C. A.; Sorensen, E. J., “Synthesis enables a structural revision of the Mycobacterium tuberculosis-produced diterpene, edaxadiene.” Chemical Science 2010, 1 (2), 202-205.
Spangler, J. E.; Sorensen, E. J., “A nature-inspired Diels-Alder reaction facilitates construction of the bicyclo 2.2.2 octane core of andibenin B.” Tetrahedron 2009, 65 (33), 6739-6745.
Schneekloth, J. S., Jr.; Kim, J.; Sorensen, E. J., “An interrupted Ugi reaction enables the preparation of substituted indoxyls and aminoindoles.” Tetrahedron 2009, 65 (16), 3096-3101.
Frie, J. L.; Jeffrey, C. S.; Sorensen, E. J., “A Hypervalent Iodine-Induced Double Annulation Enables a Concise Synthesis of the Pentacyclic Core Structure of the Cortistatins.” Organic Letters 2009, 11 (23), 5394-5397.
Tilley, S. D.; Reber, K. P.; Sorensen, E. J., “A Rapid, Asymmetric Synthesis of the Decahydrofluorene Core of the Hirsutellones.” Organic Letters 2009, 11 (3), 701-703.
Seike, H.; Sorensen, E. J., “A synthesis of the tricyclic core structure of FR901483 featuring an Ugi four-component coupling and a remarkably selective elimination reaction.” Synlett 2008, (5), 695-701.
Moreau, R. J.; Sorensen, E. J., “Classical carbonyl reactivity enables a short synthesis of the core structure of acutumine.” Tetrahedron 2007, 63 (28), 6446-6453.
Evans, M. J.; Morris, G. M.; Wu, J.; Olson, A. J.; Sorensen, E. J.; Cravatt, B. F., “Mechanistic and structural requirements for active site labeling of phosphoglycerate mutase by spiroepoxides.” Molecular Biosystems 2007, 3 (7), 495-506.
Buey, R. M.; Calvo, E.; Barasoain, I.; Pineda, O.; Edler, M. C.; Matesanz, R.; Cerezo, G.; Vanderwal, C. D.; Day, B. W.; Sorensen, E. J.; Lopez, J. A.; Andreu, J. M.; Hamel, E.; Diaz, J. F., “Cyclostreptin binds covalently to microtubule pores and lumenal taxoid binding sites.” Nature Chemical Biology 2007, 3 (2), 117-125.
Hamel, E.; Day, B. W.; Miller, J. H.; Jung, M. K.; Northcote, P. T.; Ghosh, A. K.; Curran, D. P.; Cushman, M.; Nicolaou, K. C.; Paterson, I.; Sorensen, E. J., “Synergistic effects of peloruside a and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly.” Molecular Pharmacology 2006, 70 (5), 1555-1564.
Jabbari, A.; Sorensen, E. J.; Houk, K. N., “Transition states of the retro-ene reactions of allylic diazenes.” Organic Letters 2006, 8 (14), 3105-3107.
Evans, M. J.; Saghatelian, A.; Sorensen, E. J.; Cravatt, B. F., “Target discovery in small-molecule cell-based screens by in situ proteome reactivity profiling.” Nature Biotechnology 2005, 23 (10), 1303-1307.
Related News

“I wrote a book!” Chemistry concentrators offer a few words on the senior thesis

Visiting Faculty, Students Enliven Frick Lab


