Joshua Rabinowitz
Contact:
Joshua Rabinowitz
Professor of Chemistry and the Lewis-Sigler Institute for Integrative Genomics; Director, Ludwig Princeton Branch
[email protected]
Icahn Laboratory, I241
Office: (609) 258-8985
Faculty Assistant:
Melissa Morales-Rodriguez
Faculty Assistant
[email protected]
Carl C. Icahn Laboratory, 258
609-258-7058
Research Focus
Our lab aims to achieve a quantitative, comprehensive understanding of cellular metabolism. Our motivation for studying metabolism is two-fold. From a basic science perspective, the molecular connections involved in metabolism are the best understood of any major biochemical network. Accordingly, metabolism provides a unique opportunity for quantitative analysis. From a practical perspective, derangements of metabolism are a major cause of disease, and small molecules that inhibit metabolism are the basis of many important pharmaceuticals. Accordingly, systems-level analysis of metabolism is likely to yield discoveries of medical significance.
A major barrier to understanding metabolism has been lack of appropriate tools. Our lab has developed methods for measuring a wide range of cellular metabolites using state-of-the-art mass spectrometry technology. We have used these tools to discover a novel metabolite involved in the pathogenesis of cancer (Dang et al., 2009). We have innovated approaches to quantitating metabolic fluxes by interpreting isotope-labeling data within a rigorous chemical-kinetic framework.
These analytical tools enable us to effectively re-examine long-standing and fundamental questions regarding regulation of metabolism: How are metabolite concentrations and fluxes controlled? How do microbes adapt to changing nutrient availability? How do cancer cells survive in the hypoxic environment of a tumor? In this vein, we have developed predictive, dynamic models of E. coli nitrogen metabolism (Yuan et al., 2009). Through such efforts, we are identifying key features of cellular metabolic regulation, such as competition among metabolites for enzyme active sites (Bennett et al., 2009). A future objective is to understand coordination across different nutrient systems, leading to quantitative models of the entirety of core metabolism. Such models will incorporate not just metabolite data, but also data on enzyme transcription, covalent modification, and localization. In addition to basic science utility, they will enable optimization of biofuel production.
Metabolomic tools are also opening new avenues of investigation, such as investigation into the metabolism of parasite-infected human cells. We have studied malaria and viral infections. In the latter case, we identified a dramatic up-regulation of fatty acid biosynthesis in response to viral infection. Inhibition of this pathway blocks viral replication, thereby suggesting a new strategy for antiviral therapy (Munger et al., 2008). We now aim to understand how and why viruses hijack metabolism. We also aim to identify new therapeutics based on these discoveries.
Ongoing research efforts of our fall into the following general categories:
– Technology development - Metabolic regulation in model microorganisms (Escherichia coli, Saccharomyces cerevisiae) - Biofuel production (Clostridium acetobutylicum) - Metabolic impact of viral and malaria infection - Cancer cell metabolism
All projects involve a mix of biological experiments, metabolomics, and computation.
Honors
NIH Pioneer Award (2016)
Agilent Thought Leader Award (2013)
Kavli Frontiers of Science Scholar, Kavli Foundation and National Academy of Sciences (2008)
CAREER Award, National Science Foundation (2007–2013)
Beckman Young Investigator Award, Arnold and Mabel Beckman Foundation (2005– 2008)
Medical Scientist Training Program (MSTP) Trainee, National Institutes of Health (1994–2001)
Goldwater Scholar, U. S. Federal Excellence in Education Foundation (1993–1994)
President, Phi Beta Kappa, University of North Carolina (1993–1994)
Jackson Scholar, University of North Carolina Honors Program (1990–1994)
Selected Publications
Breunig, J. S.; Hackett, S. R.; Rabinowitz, J. D.; Kruglyak, L., “Genetic Basis of Metabolome Variation in Yeast.” Plos Genetics 2014, 10 (3).
Shlomi, T.; Fan, J.; Tang, B.; Kruger, W. D.; Rabinowitz, J. D., “Quantitation of Cellular Metabolic Fluxes of Methionine.” Analytical Chemistry 2014, 86 (3), 1583-1591.
Fan, J.; Ye, J.; Kamphorst, J. J.; Shlomi, T.; Thompson, C. B.; Rabinowitz, J. D., “Quantitative flux analysis reveals folate-dependent NADPH production.” Nature 2014, 510 (7504), 298-+.
Kliegman, J. I.; Fiedler, D.; Ryan, C. J.; Xu, Y.-F.; Su, X.-y.; Thomas, D.; Caccese, M. C.; Cheng, A.; Shales, M.; Rabinowitz, J. D.; Krogan, N. J.; Shokat, K. M., “Chemical Genetics of Rapamycin-Insensitive TORC2 in S. cerevisiae.” Cell Reports 2013, 5 (6), 1725-1736.
Xu, Y.-F.; Letisse, F.; Absalan, F.; Lu, W.; Kuznetsova, E.; Brown, G.; Caudy, A. A.; Yakunin, A. F.; Broach, J. R.; “Rabinowitz, J. D., Nucleotide degradation and ribose salvage in yeast.” Molecular Systems Biology 2013, 9.
Fan, J.; Kamphorst, J. J.; Mathew, R.; Chung, M. K.; White, E.; Shlomi, T.; Rabinowitz, J. D., “Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia.” Molecular Systems Biology 2013, 9.
Koyuncu, E.; Purdy, J. G.; Rabinowitz, J. D.; Shenk, T., “Saturated Very Long Chain Fatty Acids Are Required for the Production of Infectious Human Cytomegalovirus Progeny.” Plos Pathogens 2013, 9 (5).
Tchigvintsev, A.; Tchigvintsev, D.; Flick, R.; Popovic, A.; Dong, A.; Xu, X.; Brown, G.; Lu, W.; Wu, H.; Cui, H.; Dombrowski, L.; Joo, J. C.; Beloglazova, N.; Min, J.; Savehenko, A.; Caudy, A. A.; Rabinowitz, J. D.; Murzin, A. G.; Yakunin, A. F., “Biochemical and Structural Studies of Conserved Maf Proteins Revealed Nucleotide Pyrophosphatases with a Preference for Modified Nucleotides.” Chemistry & Biology 2013, 20 (11), 1386-1398.
Guo, J. Y.; Karsli-Uzunbas, G.; Mathew, R.; Aisner, S. C.; Kamphorst, J. J.; Strohecker, A. M.; Chen, G.; Price, S.; Lu, W.; Teng, X.; Snyder, E.; Santanam, U.; DiPaola, R. S.; Jacks, T.; Rabinowitz, J. D.; White, E., “Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis.” Genes & Development 2013, 27 (13), 1447-1461.
Kamphorst, J. J.; Cross, J. R.; Fan, J.; de Stanchina, E.; Mathew, R.; White, E. P.; Thompson, C. B.; Rabinowitz, J. D., “Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids.” Proceedings of the National Academy of Sciences of the United States of America 2013, 110 (22), 8882-8887.
Grady, S. L.; Purdy, J. G.; Rabinowitz, J. D.; Shenk, T., “Argininosuccinate synthetase 1 depletion produces a metabolic state conducive to herpes simplex virus 1 infection.” Proceedings of the National Academy of Sciences of the United States of America 2013, 110 (51), E5006-E5015.
Fan, J.; Kamphorst, J. J.; Rabinowitz, J. D.; Shlomi, T., “Fatty Acid Labeling from Glutamine in Hypoxia Can Be Explained by Isotope Exchange without Net Reductive Isocitrate Dehydrogenase (IDH) Flux.” Journal of Biological Chemistry 2013, 288 (43), 31363-31369.
Commisso, C.; Davidson, S. M.; Soydaner-Azeloglu, R. G.; Parker, S. J.; Kamphorst, J. J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J. A.; Thompson, C. B.; Rabinowitz, J. D.; Metallo, C. M.; Vander Heiden, M. G.; Bar-Sagi, D., “Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells.” Nature 2013, 497 (7451), 633-+.
Reaves, M. L.; Young, B. D.; Hosios, A. M.; Xu, Y.-F.; Rabinowitz, J. D., “Pyrimidine homeostasis is accomplished by directed overflow metabolism.” Nature 2013, 500 (7461), 237-+.
Bray, K.; Mathew, R.; Lau, A.; Kamphorst, J. J.; Fan, J.; Chen, J.; Chen, H.-Y.; Ghavami, A.; Stein, M.; DiPaola, R. S.; Zhang, D.; Rabinowitz, J. D.; White, E., “Autophagy Suppresses RIP Kinase-Dependent Necrosis Enabling Survival to mTOR Inhibition.” Plos One 2012, 7 (7).
Rabinowitz, J. D.; Vastag, L., “Teaching the design principles of metabolism.” Nature Chemical Biology 2012, 8 (6), 497-501.
Xu, Y.-F.; Amador-Noguez, D.; Reaves, M. L.; Feng, X.-J.; Rabinowitz, J. D., “Ultrasensitive regulation of anapleurosis via allosteric activation of PEP carboxylase. Nature Chemical Biology 2012, 8 (6), 562-568.
Peterson, C. N.; Levchenko, I.; Rabinowitz, J. D.; Baker, T. A.; Silhavy, T. J., “RpoS proteolysis is controlled directly by ATP levels in Escherichia coli.” Genes & Development 2012, 26 (6), 548-553.
Peterson, C. N.; Levchenko, I.; Rabinowitz, J. D.; Baker, T. A.; Silhavy, T. J., “RpoS proteolysis is controlled directly by ATP levels in Escherichia coli.” Genes & Development 2012, 26 (6), 548-553.
Xu, Y.-F.; Zhao, X.; Glass, D. S.; Absalan, F.; Perlman, D. H.; Broach, J. R.; Rabinowitz, J. D., “Regulation of Yeast Pyruvate Kinase by Ultrasensitive Allostery Independent of Phosphorylation.” Molecular Cell 2012, 48 (1), 52-62.
Grady, S. L.; Hwang, J.; Vastag, L.; Rabinowitz, J. D.; Shenk, T., “Herpes Simplex Virus 1 Infection Activates Poly(ADP-Ribose) Polymerase and Triggers the Degradation of Poly(ADP-Ribose) Glycohydrolase.” Journal of Virology 2012, 86 (15), 8259-8268.
Terry, L. J.; Vastag, L.; Rabinowitz, J. D.; Shenk, T., “Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection.” Proceedings of the National Academy of Sciences of the United States of America 2012, 109 (8), 3071-3076.
Ye, J.; Mancuso, A.; Tong, X.; Ward, P. S.; Fan, J.; Rabinowitz, J. D.; Thompson, C. B., “Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation.” Proceedings of the National Academy of Sciences of the United States of America 2012, 109 (18), 6904-6909.
Reaves, M. L.; Sinha, S.; Rabinowitz, J. D.; Kruglyak, L.; Redfield, R. J., “Absence of Detectable Arsenate in DNA from Arsenate-Grown GFAJ-1 Cells.” Science 2012, 337 (6093), 470-473.
Doucette, C. D.; Schwab, D. J.; Wingreen, N. S.; Rabinowitz, J. D., “alpha-ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition.” Nature Chemical Biology 2011, 7 (12), 894-901.
Kamphorst, J. J.; Fan, J.; Lu, W.; White, E.; Rabinowitz, J. D., “Liquid Chromatography-High Resolution Mass Spectrometry Analysis of Fatty Acid Metabolism.” Analytical Chemistry 2011, 83 (23), 9114-9122.
Related News
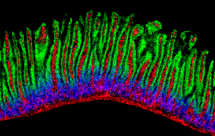
Rabinowitz/Raphael labs map tissue metabolism at cellular resolution

Princeton joins new cancer research hub established with gift from Weill Family Foundation