Chang Lab identifies protein directing the evolution of sleep
It turns out there is more than one missing link distinguishing lower-order organisms from higher-order, and sleep behaviors may be one of them.
Through funding from the National Institutes of Health (NIH), researchers in the Chang Lab have discovered a copper-transport mechanism in the brain that enables advanced stages of sleep, like Rapid Eye Movement (REM) in which dreaming occurs, that is not activated in less complex organisms. The demarcation at which it becomes active lines up perfectly with the evolution from aquatic- to land-based animals.
REM sleep has previously been found in reptiles but not beneath this taxonomic class.
The Chang Lab’s findings have broad implications for evolutionary biology and neuroscience by underscoring the central role of copper in animal physiology.
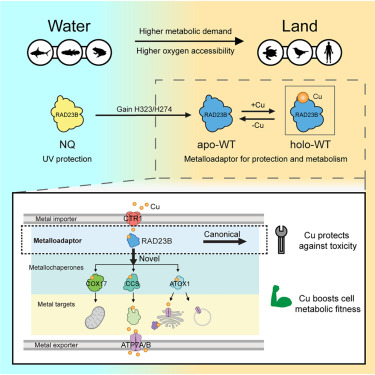
The lab's paper, "RAD23B acquires a copper metalloadaptor function in amphibian-to-reptile evolution to increase metabolism and regulate genomic integrity," was recently published in Molecular Cell.
“What we found is that the sleep-wake cycle could be altered by metal nutrients like copper, and that it’s important for not just sleep but stages of sleep. So we think the nutrient itself could be the elemental origin for how sleep evolved,” said Christopher Chang, the Edward and Virginia Taylor Professor of Bioorganic Chemistry. “When you transition life from water to land, then you start to develop stages of sleep. This really is the first step to understanding its molecular basis.
“It’s also a perfect example of why we need to do basic research,” said Chang on the value of Blue Sky thinking. “Why is there so much of this metal element in this part of the body, the brain? We asked this simple question with childlike curiosity. And then we proceeded to study it piece by piece, just like a detective. We observed something in the natural world and then we sought to understand it.”
It all starts with a small but mighty nucleus in the brain stem called the locus coeruleus (LC) that concentrates more copper than any other part of the body. Researchers in the Chang Lab wanted to know why. The LC is crucial to the sleep-wake cycles, so they began their investigation there. In the process they uncovered a DNA repair protein called RAD23B that has a gain of function at this evolutionary threshold to become a master regulator of copper, which scientists call a metalloadaptor. Prior to this, sleep is essentially binary for lower-order organisms: there is only sleep and wake, no REM.
“For an animal to adapt to a new environment, there are always these small switches,” said Tong Xiao, first author and a staff scientist in the Chang Lab. “An animal will gain a certain function and that is because of an adaptation during evolution. That’s how these types of mutations accumulate.”

Christopher Chang, the Edward and Virginia Taylor Professor of Bioorganic Chemistry and P.I. on this research.
“So we found one of these small switches, because to be able to sleep for a chunk of time is essential for the metabolic fitness of the brain,” she said, adding that the brains of more complex animals have a higher metabolic demand.
“The really key thing is how the LC changes evolutionarily from the lower organisms to the higher, from fish to mammal, from water to land. We looked at the protein structure of RAD23B and we saw this amino acid change and I thought, well there is something here. And that’s how we saw this switch.”
The lab’s research, RAD23B acquires a copper metalloadaptor function in amphibian-to-reptile evolution to increase metabolism and regulate genomic integrity, was published last month in Molecular Cell.
HOW IT WORKS
The regulation of copper is essential to maintain biological homeostasis. The body cannot manufacture this metal, so it comes in through the diet and is disbursed through the body by various cellular transport mechanisms. The brain’s LC marks the largest of these areas of concentration.
The Chang Lab found that RAD23B in the LC acts as a central depot, directing the transport of copper to the correct destination by binding copper and then shuttling it to wherever it needs to go in the cell. This protein is extant in all living organisms, from yeast to human beings, but it does not have this shuttle function—it cannot bind copper—until it reaches the level of land-dwelling organisms.

Tong Xiao, first author on the journal paper and a staff scientist in the Chang Lab.
Under this mechanism, RAD23B gains an allosteric copper-binding site through two histidine amino acid residues to enable the transfer of copper from the cells’ universal transporter uptake protein, called CTR1, to all known copper shuttle pathways. It acts like a quarterback on a football team masterminding the movement downfield of the football—or, in this case, copper—to other players on the team.
“You have two coincident things occurring: REM stages of sleep occur at this really important transition from life in water to life on land,” said Chang. “And then you also have this single protein where two amino acids change to be able to bind copper at that exact same stage in evolution,” said Chang. “So it suggests that this is the molecular target for copper.”
Researchers said the paper lays down a path for considerable follow-up research, specifically about the impact of copper on sleep and neurodevelopment as well as copper homeostasis.
“There’s so much more we can do with this. This is the best part of science – you can just keep going,” said Xiao. “We will definitely try to see if we can make animals able to tolerate copper better by making similar genetic mutations to what we saw in this research.
“But overall, we’re looking for similar copper-binding motifs in other proteins, as well.”
This research was supported by funding from the National Institutes of Health (NIH).
