Chemists join forces to develop cheap, complementary method for classic reaction
A team of researchers at Princeton University, the Massachusetts Institute of Technology and Merck & Co. has developed a cost-effective and complementary approach to a fundamental chemical reaction, known as a C-N bond coupling, which is a staple of modern drug development.
Published today in the journal Science, the reaction provides a direct route to compounds called anilines, an extremely common structure in medicinal and industrially relevant agents, without the expense of ligands on transition metal catalysts required by traditional methods.
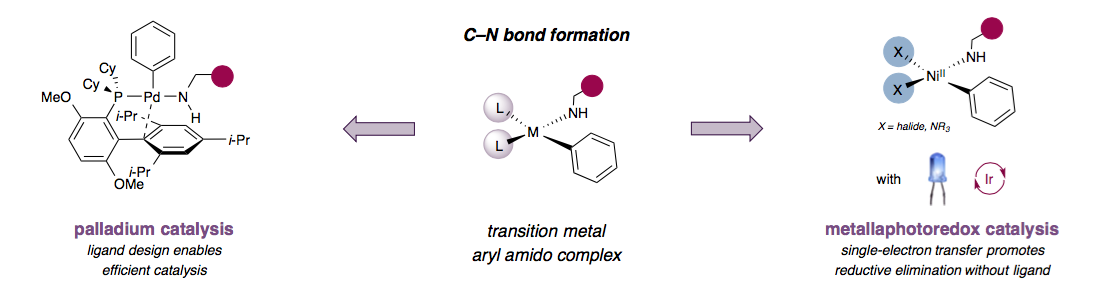
Image credit: AAAS/Science
“What we bring to the table in this paper is a completely new mechanism to perform this reaction,” said David W. MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry and a corresponding author on the paper.
Whereas established C-N bond couplings rely on tailored ligands to destabilize the metal catalyst and drive the reaction forward, the MacMillan lab proposed that light and a photoredox catalyst could instead take on that role. Once initial results confirmed their hypothesis, they reached out to Stephen Buchwald, the Camille Dreyfus Professor of Chemistry at MIT, whose group had worked extensively in C-N bond couplings, also known as Buchwald-Hartwig aminations.
“While we felt we knew a lot about photoredox, we felt we really needed to work with someone that understood C-N bond formation,” MacMillan said. “Buchwald will undoubtedly win a Nobel Prize for C-N couplings and as such he was the perfect person and group to ask to collaborate on this study. We were lucky he agreed to do so.”
Image credit: Emily Corcoran
Through teleconferences and email, researchers in both labs worked to optimize the method. They found that the reaction only needed tiny amounts of photoredox catalyst in conjunction with a very cheap and simple nickel catalyst to give efficient yields for a range of coupling partners.
“What I think is very intriguing and cool about this project is that it’s almost the opposite of the way we usually think,” said Michael Pirnot, a postdoctoral researcher in the Buchwald group and co-author on the paper, “where instead of trying to find a specialized ligand, you can run the reaction without adding any sort of engineered ligand at all.”
An attractive feature of the reaction is that researchers largely employ a mild base, which is helpful when performing chemistry on delicate substrates. Additionally, the homogeneity of the reaction makes it especially amenable to flow conditions, in which the reaction solution is steadily pumped through a reactor. It has been well established that photoredox catalysis can benefit dramatically with faster reaction times in flow given the increased solution concentration and exposure to light.
Finally, the researchers teamed up with Merck & Co. to test the utility of their photoredox C-N coupling method on a so-called ‘informer’ library that has been used to benchmark other methods. Using this high-throughput technique, the researchers found that 78 percent of these drug-like compounds in the library underwent successful coupling, suggesting that their protocol would find broad applicability in a medicinal chemistry setting.
“That was one of the most enjoyable parts for me, seeing how other people could benefit from our work,” said Emily Corcoran, a postdoctoral researcher in the MacMillan lab and lead author on the article.
Like the merger of photoredox and nickel catalysis in the reaction, through collaboration the researchers were able to achieve more than they could have on their own. “You cannot overstate the value of collaboration when it is done well,” MacMillan said.
Read the full paper here:
Corcoran, E. B.; Pirnot, M. T.; Lin, S.; Dreher, S. D.; DiRocco, D. A.; Davies, I. W.; Buchwald, S. L.; MacMillan, D. W. C. “Aryl Amination using Ligand-Free Ni(II) Salts and Photoredox Catalysis.” Science. Published online on June 23, 2016 in First Release Science Papers.
This work was supported by the National Institutes of Health grants GM58160, RO1-GM078201-05, and postdoctoral fellowship GM113311 awarded to M.T.P.
