Hyster Lab Receives Foundation Grant to Address HIV Therapy
Todd Hyster, an assistant professor in the Department of Chemistry, has received a grant from the Bill & Melinda Gates Foundation to develop improved, cost-effective syntheses of molecules essential to global health advances, particularly in the fight against AIDS in developing countries where the cost of therapies is an enduring concern.

Photo by C. Todd Reichart
The product of conversations that began a year ago, the grant will focus on Darunavir, an antiviral used in the treatment of HIV. Researchers will prepare the core structure found in the drug molecule using biocatalysis, an approach that relies on biological systems to catalyze, or drive, molecular reactions.
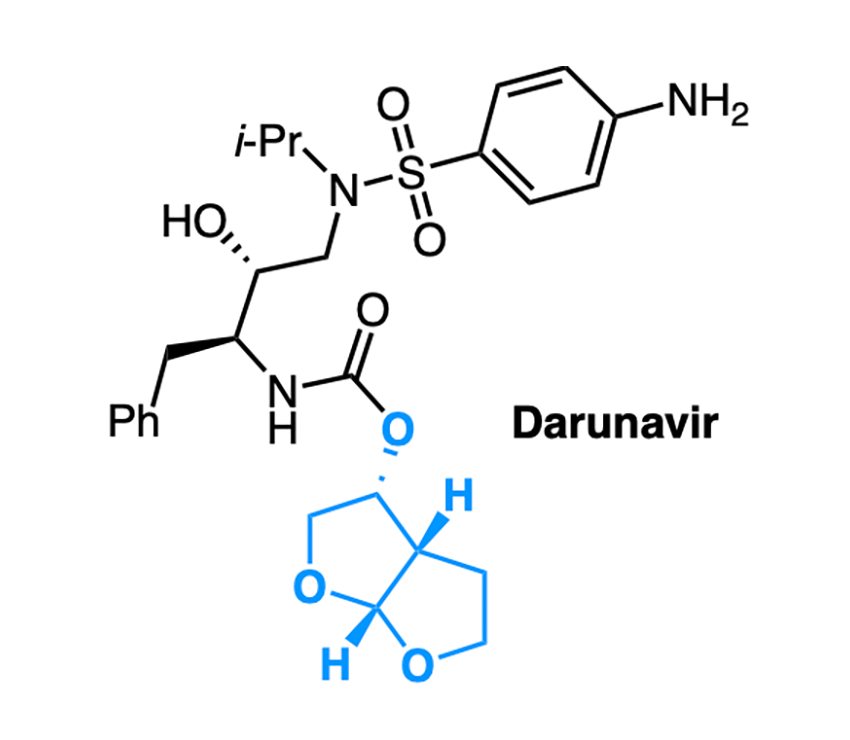
Image courtesy of Yuxuan Ye
Enzymes will serve as the vehicle for the catalysis. Naturally occurring and therefore environmentally favorable, enzymes carry out synthetic transformations with levels of selectivity that might otherwise be very difficult to achieve in classical synthesis. Because the enzymes used will be non-proprietary, the costs of drug development should be further reduced.
“I am incredibly excited,” said Hyster of the one-year grant that just launched. “The Gates Foundation is famous for identifying important problems, and I think the validation of being able to apply an area that I’m passionate about to helping solve one of those problems is incredibly rewarding.
“The foundation provided us with molecules of value and we came up with some syntheses that relied on reactions already known in nature. If you look at how biocatalysis is used today, you find that engineered enzymes are often used by the pharmaceutical industry. But the cost of developing these processes can be expensive,” Hyster added. “We’re looking to develop a more efficient process.”
The Hyster lab started their research by identifying ways to build the molecular architecture of the drug, focusing in on that part of the process where an enzyme might be ideally suited for a particular chemical transformation.
Ultimately, researchers chose the technique of “setting” molecular stereocenters with a ketoreductase (an enzyme that catalyzes the reduction of a ketone) in a process called dynamic kinetic resolution. Setting the stereocenters gives researchers greater control over the drug molecule’s properties.
Lab members will make the starting material for the process themselves. Later, they’ll work with a protein company that provides the ketoreductase, or “k-reds,” so that a selective enzyme can be identified.
Yuxuan Ye, a postdoctoral fellow in the Hyster lab, described three general steps to the research; each step is a discrete transformation turning one molecule into the next as the process moves along. The first step is to synthesize the “precursor,” an organic compound that contains a ketone – the functional group that participates in the enzymatic reaction; the second step is where the key biocatalysis takes place; the third is another chemical synthesis step that finishes the synthesis. Hyster’s research will use enzymes in the second step to perform the ketone reduction – which will take advantage of the high selectivity of the enzyme – to set the stereocenters.
Stereochemistry is often one of the most challenging aspects of organic synthesis. Paul Riehl, a postdoctoral fellow in the Hyster lab, explained.

Photo by C. Todd Reichart
“When a molecule has a stereocenter and a subsequent reaction introduces a new one, the 3D shape of the first can have an impact on the second,” Riehl said. “Since enzymes are comparatively large molecules with many stereocenters, they can have exceptionally high levels of stereochemical control when they act as catalysts.”
Ye added, “During the enzymatic dynamic kinetic resolution process, out of four possible stereoisomers of the product, we could access the exact one we want. This is a very efficient synthesis.”
Hyster is hopeful the research will inspire the use of enzymes in these types of syntheses for companies seeking greater efficiencies and more environmentally friendly ways of achieving them.
“I think the Gates Foundation is very much interested in taking fundamental advances and making sure that they translate into some sort of deliverable that would be valuable for the world,” said Hyster. “Once we establish the route, there should be a lot of interest in identifying partners so we can begin to think about scaling.”
The Bill & Melinda Gates Foundation is a global leader in seeking solutions to intractable problems. One foundation goal is accelerating the decline in HIV infection worldwide and ensuring expanded and simplified HIV treatments, according to the foundation website. Some 37 million people around the globe are living with HIV, 70% of whom are in Sub-Saharan Africa. The foundation celebrates the 20th Anniversary of its founding this year.
This research is supported by the Bill & Melinda Gates Foundation grant number INV-005885, “Streamlined Synthesis of Darunavir using Biocatalytic Cascades.”
