Craig Townsend
Craig Townsend
Wed, Sep. 29, 2021, 4:00pm
Taylor Auditorium
Host: G.S.O. and the Seyedsayamdost Lab
Genus Enediynei est omne divisum in partes tres
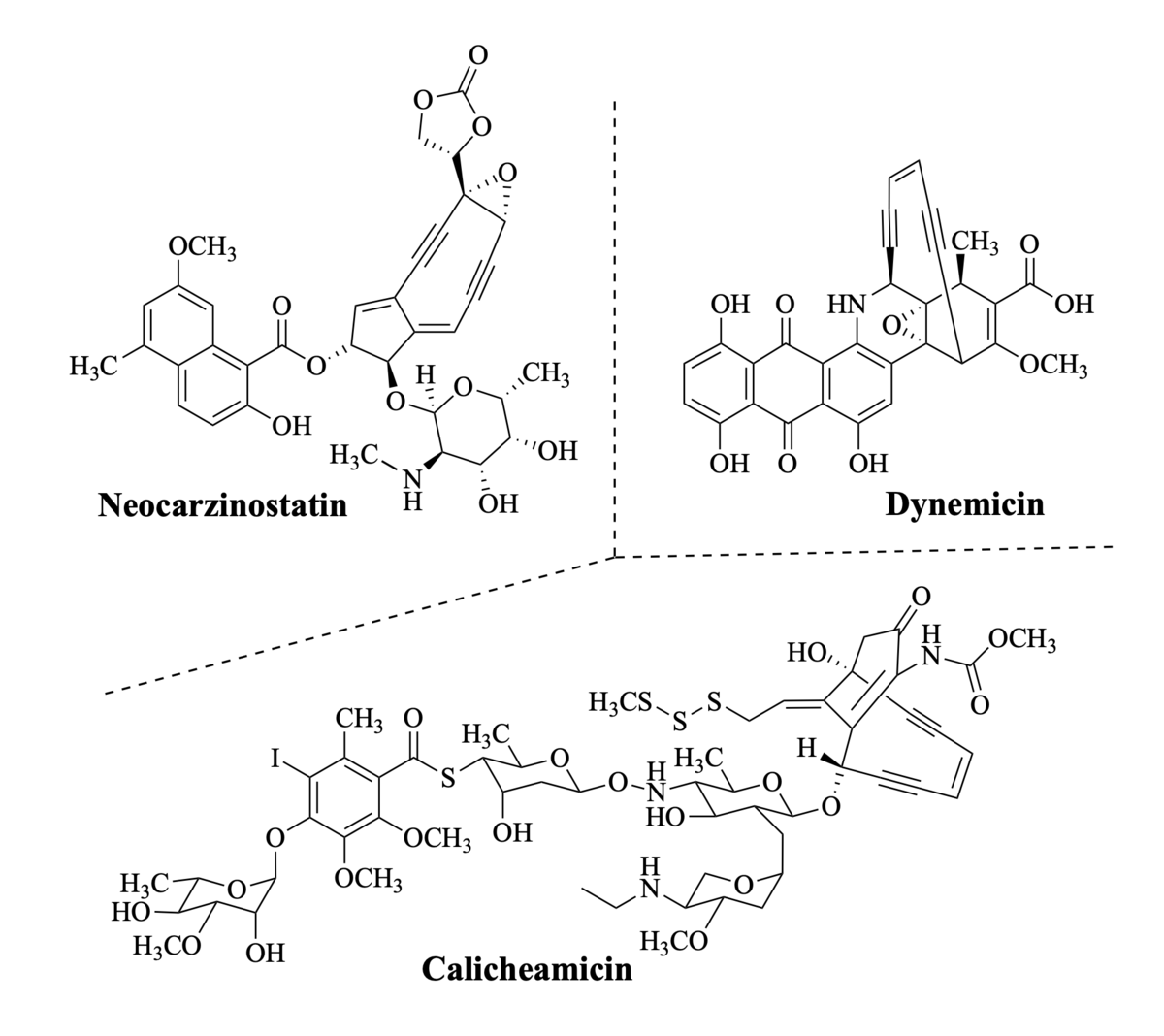
Like ancient Gaul for Julius Caesar, all enediyne natural products can be divided into three parts. These microbial metabolites are of interest for their mechanism of potent DNA cleaving ability and their emblematic structures. They also constitute a classic unsolved problem in natural product biosynthesis. Clusters of genes that ultimately encode their syntheses comprise 60-75 members and portend the complexity of these mostly unknown processes—yet they all arise from a common, seemingly unrelated linear precursor. In this lecture gene inactivation, chemical synthesis, structure determination, enzymology and chemical model reactions will be applied to decrypt the heterodimeric group of enediynes exemplified by Dynemicin.
Background references:
- Belecki, K.; Townsend, C. A. “Biochemical Determination of Enzyme-bound Metabolites: Preferential Accumulation of a Programmed Octaketide on the Enediyne Polyketide Synthase CalE8.” J. Am. Chem. Soc. 2013, 135, 14339-14348.
- Cohen, D. R.; Townsend, C. A. “A Dual Role for a Polyketide Synthase in Dynemicin Enediyne and Anthraquinone Biosynthesis.” Nat. Chem. 2018, 10, 231-236.
