Dan Weix
Dan Weix
Tue, Nov. 18, 2014, 4:30pm - 6:00pm
Frick Chemistry Laboratory, Taylor Auditorium
Host: Brad Carrow
The Power of Two: Multimetallic Approaches to Cross-Electrophile Coupling
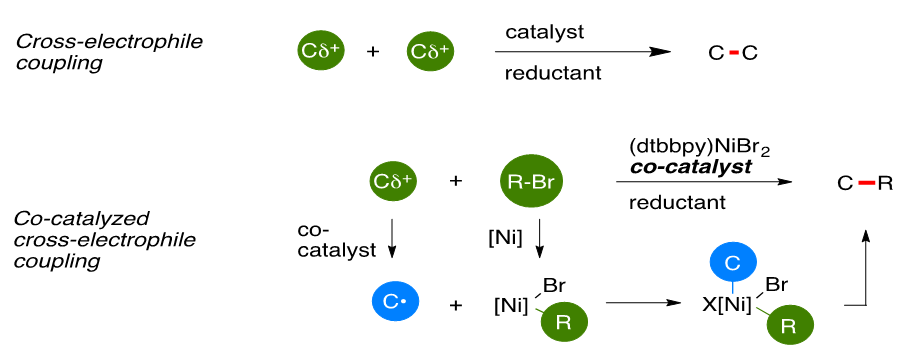
Cross-electrophile coupling is the broadly-defined union of two different electrophiles through transition-metal catalysis under reducing conditions. By avoiding pre-formed carbon nucleophiles, cross-electrophile coupling avoids the challenges of substrate availability and functional group compatibility associated with these organometallic reagents. Substitution of a second electrophile for the organometallic partner in cross-couplings offers the potential to dramatically increase the number and types of molecules that can be easily made because of the large number of commercially available carbon electrophiles (>1 million R-X vs. ~5 thousand R-B(OH)2) and the low cost of all components. We have recently demonstrated reactions that couple organic halides with enones, aryl halides with alkyl halides, and acid chlorides and thioesters with alkyl halides, demonstrated their high functional-group compatibility, and shed light on certain reactions are cross-selective and others are not.
This talk will introduce our current understanding of the origin of selectivity in cross-electrophile coupling reactions and introduce the a powerful, new concept for broadening substrate scope: co-catalysis. Three different co-catalysts will be discussed, each allowing access to different substrate classes.