BMS Symposium Featuring Jin-Quan Yu
Bristol Myers Squibb Symposium
Tue, May. 2, 2023, 3:30pm
Taylor Auditorium, Frick Chemistry Lab B02
Host: Robert Knowles
3:30PM – Bruce Ellsworth – Synthesis in drug discovery, and the impact of increasing sp3 character on compound quality
This lecture will highlight the role of increasing sp3 character and chirality in molecular design to identify high quality compounds for clinical advancement to treat diseases. Two case studies will be discussed wherein initial compounds were highly lipophilic and aromatic. Efforts to reduce aromaticity (sp2 character, referred to in the literature as “escape from flatland”) and to reduce lipophilicity and an increase in chirality often results in more highly selective compounds. The presentation will focus on the discovery of these interesting structures, development of chiral synthetic routes, and the clinical candidates that result from such efforts.
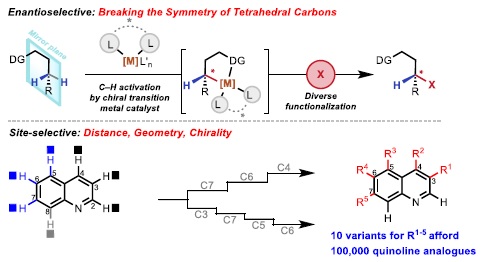
4:30PM – Jin-Quan Yu – 20-Year Dancing with Palladium and C–H bonds: From Curiosity to Industrialization
The widespread presence of C–H bonds at various sites of synthetic substrates renders C–H activation the most powerful platform for developing catalytic reactions for synthesis. Both the measurement for success and approaches for addressing this synthetic problem are distinct from the long-pursued methane oxidation or alkane functionalizations, where one extremely efficient reaction of one particular C–H bond is needed. In contrast, to realize the full potential of C–H activation for synthesis, four fundamental challenges must be addressed: developing diverse carbon-carbon and carbon-heteroatom bond forming reactions of diverse poorly reactive native substrates (ReactivitY); enantioselective C–H activation reactions via asymmetric metalation of C–H bonds (EnantioselectivitY); site selective metalation and functionalization of remote C–H bonds (Site-selectivitY); achieving catalytic cycles using sustainable oxidants such as molecular oxygen, aqueous hydrogen peroxides as the terminal oxidants (SustainabilitY). Despite a century-long efforts on understanding and developing C–H activation reactions, seeking solutions to these problems has met with limited success due to: lack of reactivity of broadly useful substrates; lack of well-defined stereomodel for enantioselective metal insertion; lack of predictable approaches for controlling site selectivity as majority of C–H bonds are electronically and sterically similar, hence difficult to distinguish; and most importantly, lack of ligands that can accelerate C–H activation reactions. By combining the weak coordination (entropy) from substrates and ligand acceleration (enthalpy), we have made substantial progress towards addressing these four challenges. Most notably, eight generations of bi-functional ligands (MPAA, APAQ, APAO, MPAAm, MPAThio, Pyridone, Pyridine-Pyridone, Carbox-Pyridone) have been developed to enable a wide range of enantioselective1-6 and site-selective7-10 C–H activation reactions of diverse classes of native substrates. Most recently, we have realized C–H hydroxylation using molecular oxygen or aqueous hydrogen peroxide as the terminal oxidants, paving the way for large-scale industrialization.11,12