Josep Cornella
Bismuth Redox Catalysis
Wed, Nov. 30, 2022, 4:30pm
Taylor Auditorium, Frick Chemistry Lab B02
Host: SILS
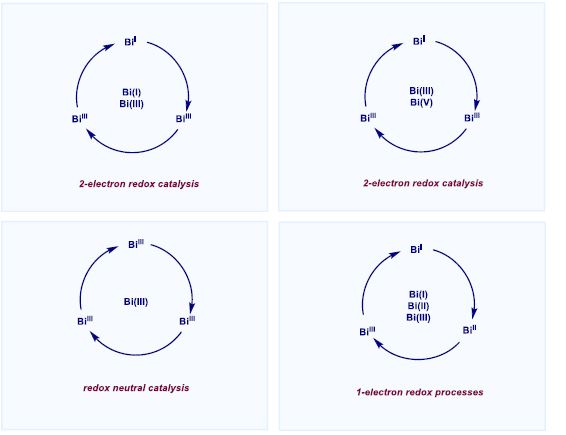
The ability of the main group element bismuth (Bi) to maneuver between different oxidation states in a catalytic redox cycle will be presented. We will show how Bi mimics the canonical organometallic steps of a transition metal, thus challenging the current dogmas of catalysis.[1] A series of Bi complexes capable of two-electron redox catalysis have been unlocked and applied in various contexts of catalysis for organic synthesis. For example, capitalizing on the Bi(III)/Bi(V) redox pair, we have developed a catalytic protocol for the C‒F[2] and C‒OTf [3] bond formation from aryl boronic esters. On the other hand, a low-valent redox manifold based on Bi(I)/Bi(III) enabled catalytic transfer hydrogenation[4], catalytic decomposition of inert nitrous oxide (N2O)[5] and catalytic hydrodefluorination of C(sp2)‒F bonds.[6] Recently, we have shown that one-electron pathways are also accessible, thus enabling a unique platform for synthesis based on SET processes through the triad Bi(I)/Bi(II)/Bi(III).[7] Finally, we will also show how redox-neutral catalytic pathways can unlock novel organic transformations via canonical organometallic steps.[8] For all methodologies, a combination of rational ligand design with an in depth analysis of all the catalytic steps proved crucial to unfold the catalytic properties of such an intriguing element of the periodic table.