Tanja Cuk
Tanja Cuk
Mon, Oct. 10, 2016, 4:30pm - 6:00pm
Frick Chemistry Laboratory, Taylor Auditorium
Host: Andrew Bocarsly
Observing Molecular Dynamics of Catalysis at Solid-Liquid Interfaces
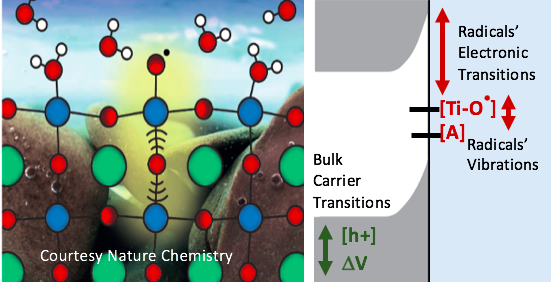
The transformation of charge into new molecular bonds guides catalysis and is at the heart of current efforts to create solar-to-fuel systems. My lab leads an effort in observing the molecular dynamics of catalysis at the solid-liquid interface by merging multi-color, transient (optical, mid-infrared, & x-ray) spectroscopy with photo-electrochemistry of the water oxidation reaction. The research identifies the essential intermediates of the reaction at the molecular level, their dynamic interaction with reactant molecules, their formation from charge carriers, and their motion along the interface. Thus far, three breakthroughs were made regarding the catalytic intermediates required to form the first bond of the water oxidation cycle (e.g., O-O), or those nascent upon charge transformation at the catalytic surface: (1) The detection of the oxyl radical (Ti-O·) by its unique, sub-surface vibration at the n-SrTiO3/aqueous interface using ultrafast mid-infrared spectroscopy and theory1. The oxyl’s vibration couples to water librations, which means that it excites H-bond breaking events requisite for bond formation; this important, dynamic interaction between a catalytic intermediate and reactant molecules had yet to be observed. (2) The observation of the rates at which these radicals form at the aqueous interface out of charge carriers in solid-state bands, which led to a proposed (non-adiabatic) kinetic mechanism for their capture2. (3) The increase of interfacial charge mobility that results from reaction intermediates created at the n-GaN/aqueous interface by transient optical diffraction3. The future work in my laboratory is to unify and further the perspectives of the solid-liquid interface that these breakthroughs offer. It is to create a dynamic picture of how product evolution emerges from catalytic intermediates on a variety of materials, for different device architectures, and for diverse reactions.
