
Clarence E. Schutt
Contact:
Schutt Group Website
Clarence E. Schutt
Professor of Chemistry, Emeritus
[email protected]
Frick Laboratory, 361
609-258-4435
Research Focus
The basis for movement and force generation in muscle and non-muscle cells is the actin-based microfilament system. Actin is a 43 kilo-dalton protein that has been highly conserved since the dawn of the eukaryotes over a billion years ago. Primordial precursor forms of actin and tubulin exist in bacteria, pointing to an even earlier origin for polymerizing proteins as generators of movement. Its emergence at the same time as the eukaryotic cell membrane, replacing the rigid cell walls of prokaryotes, enabled cells to exhibit a wide variety of dynamic shape variation at the cell edge. These movements are the structural basis for cell migration, tension generation, engulfment of other organisms, muscle contraction, and various modes of nutrient transport. Perhaps the most dynamic manifestation of the versatility of the actin microfilament system is the human nervous system, where cycles of polymerization and depolymerization of actin are an essential element of neural plasticity.
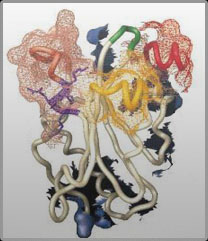
Schutt’s laboratory has used x-ray crystallography, selected-site mutagenesis, and other biophysical techniques to reveal the structures and roles of actin-binding proteins. This large family of proteins, including profilin, gelsolin, actin depolymerization protein, tropomyosin, and myosin, controls the dynamic organization of the actin microfilament system. Recently, Schutt has become interested in the structural biology of human neuro-developmental disorders, such as autism, where genomic variation in genes for key actin-binding proteins are well-correlated with dysfunctional synaptic connections. The architectonic challenge of bridging human behavior to molecular changes in the human brain must involve a full realization of the potential of the actin microfilament system to continuously reorganize into functional structures at cell edge and inwards towards the cell nucleus.
Honors
Distinguished Lectureship, University of Pennsylvania (2013)
Honorary Professorship, San Marcos University (Lima, Peru, 2006)
Ph.D. Honoris Causa, Stockholm University (1988)
Selected Publications
Lovelace, J. J.; Murphy, C. R.; Daniels, L.; Narayan, K.; Schutt, C. E.; Lindberg, U.; Svensson, C.; Borgstahl, G. E. O., “Protein crystals can be incommensurately modulated.” Journal of Applied Crystallography 2008, 41, 600-605.
Grenklo, S.; Hillberg, L.; Rathje, L.-S. Z.; Pinaev, G.; Schutt, C. E.; Lindberg, U.,” Tropomyosin assembly intermediates in the control of microfilament system turnover.” European Journal of Cell Biology 2008, 87 (11), 905-920.
Lindberg, U.; Schutt, C. E.; Goldman, R. D.; Nyakern-Meazza, M.; Hillberg, L.; Rathje, L.-S. Z.; Grenklo, S., “Tropomyosins Regulate the Impact of Actin Binding Proteins on Actin Filaments.” Tropomyosin 2008, (644), 223-231.
Schueler, H.; Karlsson, R.; Schutt, C. E.; Lindberg, U., “The Connection Between Actin ATPase and Polymerization.” Aspects of the Cytoskeleton 2006, (37), 49-66.
Hillberg, L.; Rathje, L. S. Z.; Nyakern-Meazza, M.; Helfand, B.; Goldman, R. D.; Schutt, C. E.; Lindberg, U., “Tropomyosins are present in lamellipodia of motile cells. “European Journal of Cell Biology 2006, 85 (5), 399-409.
Lovelace, J. J.; Narayan, K.; Chik, J. K.; Bellamy, H. D.; Snell, E. H.; Lindberg, U.; Schutt, C. E.; Borgstahl, G. E. O., “Imaging modulated reflections from a semi-crystalline state of profilin : actin crystals.” Journal of Applied Crystallography 2004, 37, 327-330.
Narayan, K.; Chumnarnsilpa, S.; Choe, H.; Irobi, E.; Urosev, D.; Lindberg, U.; Schutt, C. E.; Burtnick, L. D.; Robinson, R. C., “Activation in isolation: exposure of the actin-binding site in the C-terminal half of gelsolin does not require actin.” Febs Letters 2003, 552 (2-3), 82-85.
Chakrabarti, R.; Schutt, C. E., “Novel sulfoxides facilitate GC-rich template amplification.” Biotechniques 2002, 32 (4), 866-+.
Springs, S. L.; Bass, S. E.; Bowman, G.; Nodelman, I.; Schutt, C. E.; McLendon, G. L., “A multigeneration analysis of cytochrome b(562) redox variants: Evolutionary strategies for modulating redox potential revealed using a library approach.” Biochemistry 2002, 41 (13), 4321-4328.
Nyman, T.; Page, R.; Schutt, C. E.; Karlsson, R.; Lindberg, U., “A cross-linked profilin-actin heterodimer interferes with elongation at the fast-growing end of F-actin.” Journal of Biological Chemistry 2002, 277 (18), 15828-15833.
Nyakern-Meazza, M.; Narayan, K.; Schutt, C. E.; Lindberg, U., “Tropomyosin and gelsolin cooperate in controlling the microfilament system.” Journal of Biological Chemistry 2002, 277 (32), 28774-28779.
Nyman, T.; Schuler, H.; Korenbaum, E.; Schutt, C. E.; Karlsson, R.; Lindberg, U., “The role of MeH73 in actin polymerization and ATP hydrolysis.” Journal of Molecular Biology 2002, 317 (4), 577-589.
Chakrabarti, R.; Schutt, C. E., “The enhancement of PCR amplification by low molecular weight amides.” Nucleic Acids Research 2001, 29 (11), 2377-2381.
Chakrabarti, R.; Schutt, C. E., “The enhancement of PCR amplification by low molecular-weight sulfones.” Gene 2001, 274 (1-2), 293-298.
Bowman, G. D.; Nodelman, I. M.; Hong, Y.; Chua, N. H.; Lindberg, U.; Schutt, C. E., “A comparative structural analysis of the ADF/cofilin family.” Proteins-Structure Function and Bioinformatics 2000, 41 (3), 374-384.
Schutt, C. E.; Lindberg, U., “The new architectonics: An invitation to structural biology.” Anatomical Record 2000, 261 (5), 198-215.
Schuler, H.; Lindberg, U.; Schutt, C. E.; Karlsson, R., “Thermal unfolding of G-actin monitored with the DNase I-inhibition assay – Stabilities of actin isoforms.” European Journal of Biochemistry 2000, 267 (2), 476-486.
Schuler, H.; Nyakern, M.; Schutt, C. E.; Lindberg, U.; Karlsson, R., “Mutational analysis of arginine 177 in the nucleotide binding site of beta-actin.” European Journal of Biochemistry 2000, 267 (13), 4054-4062.
Schuler, H.; Schutt, C. E.; Lindberg, U.; Karlsson, R., “Covalent binding of ATP gamma S to the nucleotide-binding site in S14C-actin.” Febs Letters 2000, 476 (3), 155-159.
Bowman, G. D.; Nodelman, I. M.; Levy, O.; Lin, S. L.; Tian, P.; Zamb, T. J.; Udem, S. A.; Venkataraghavan, B.; Schutt, C. E., “Crystal structure of the oligomerization domain of NSP4 from rotavirus reveals a core metal-binding site.” Journal of Molecular Biology 2000, 304 (5), 861-871.
Schuler, H.; Korenbaum, E.; Schutt, C. E.; Lindberg, U.; Karlsson, R., “Mutational analysis of Ser14 and Asp157 in the nucleotide-binding site of beta-actin.” European Journal of Biochemistry 1999, 265 (1), 210-220.
Nodelman, I. M.; Bowman, G. D.; Lindberg, U.; Schutt, C. E., “X-ray structure determination of human profilin II: A comparative structural analysis of human profilins.” Journal of Molecular Biology 1999, 294 (5), 1271-1285.
Korenbaum, E.; Nordberg, P.; Bjorkegren-Sjogren, C.; Schutt, C. E.; Lindberg, U.; Karlsson, R., “The role of profilin in actin polymerization and nucleotide exchange.” Biochemistry 1998, 37 (26), 9274-9283.
Schutt, C. E.; Lindberg, U., “Muscle contraction as a Markov process I: energetics of the process.” Acta Physiologica Scandinavica 1998, 163 (4), 307-323.
Page, R.; Lindberg, U.; Schutt, C. E., “Domain motions in actin.” Journal of Molecular Biology 1998, 280 (3), 463-474.
Springs, S. L.; Bass, S. E.; Bowman, G.; Nodelman, I.; Schutt, C. E.; McLendon, G. L., “A multigeneration analysis of cytochrome b(562) redox variants: Evolutionary strategies for modulating redox potential revealed using a library approach.” Biochemistry 2002, 41 (13), 4321-4328.
