

Paul Chirik
Contact:
Paul Chirik
Edwards S. Sanford Professor of Chemistry
[email protected]
Frick Laboratory, 292
609-258-4130
Faculty Assistant:
Julia Kazmierczak
Faculty / Grants Assistant
[email protected]
Frick Laboratory, 289
609-258-4883
Research Focus
Catalysis is at the core of sustainable chemistry. Many catalytic processes, however, rely on elements that are expensive, in short supply or have a significant environmental footprint associated with their extraction and isolation. The Chirik group is exploring “modern alchemy” for sustainable chemistry – a concept defined as using ligand design to transmute the function of an earth abundant metal to mimic or ideally surpass the performance of more exotic precious elements.
Our approach is multidisciplinary, spanning the traditional areas of organic and inorganic chemistry. At the core of our work is understanding and manipulating electron flow in first row transition metal compounds. We integrate modern spectroscopic and theoretical methods to accomplish these objectives. In one limit, oxidation and reduction chemistry occurs cooperatively between the metal and the supporting ligand. This concept of “redox active” ligands has resulted in new base metal catalysts for the asymmetric hydrogenation of alkenes as well as the hydrosilylation and hydroboration of olefins.
In each case, the base metal catalysts offer unique function over established precious metals catalysts.
Our catalyst development efforts are often paired with industrial partners as our catalysts find relevance to problems in the pharmaceutical, flavor and fragrance, petrochemical and silicones industries. In chemistry unique to these electronic structures, we have also discovered unprecedented cycloaddition reactions that overcome the constraints of orbital symmetry and enable a new, thermal route to various cyclobutane derivatives. The applications of these methods to various synthetic targets are under investigation.
An alternative approach is confining redox changes to the base metal. In this so-called strong field limit, the base metal catalysts are sufficiently electron rich to enable the activation and functionalization of ubiquitous but otherwise kinetically inert C-H bonds. A general, cobalt-catalyzed method for C-H borylation has been discovered with distinct advantages over existing precious metal catalysts. The application of this process to various synthetic challenges are currently under investigation. More broadly, our group actively explores how manipulation of electronic structure can be applied to new problems in catalysis.
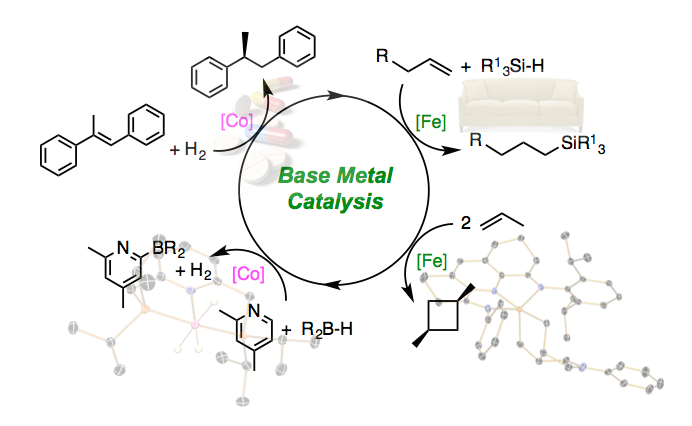
Diagram by Jon Darmon
A second area of long-standing interest is the fixation of molecular nitrogen, N2. While the industrial Haber-Bosch process has had a transformative impact on society – enabling food production for approximately half of the world’s population and accounting for half of the nitrogen in the body – the fossil fuel inputs and carbon footprint associated with this reaction inspire the search for alternatives. Our laboratory is developing new routes to N-H and N-C bond forming reactions using molecular nitrogen as the building block. If successful, these methods would either circumvent the Haber-Bosch process or result in ammonia synthesis methods that are compatible with renewable hydrogen. To accomplish these objectives, we study early transition metal complexes that by virtue of their designed coordination environment, activate or even cleave the strong NN bond in N2. One particularly notable process is ligand-induced N2 bond cleavage, where various ureas, oxamides, formates and amines have been synthesized from N2 and CO – diatomic molecules with the two strongest bonds in chemistry.
Honors
- Elected Fellow, American Association for the Advancement of Science (2022)
- Gabor Somorjai Award for Creative Research in Catalysis (2021)
- Linus Pauling Medal (2020)
- Rylander Award in Catalysis Sponsored by BASF (2020)
- Eni Award, Advanced Environmental Solutions Prize (2019)
- ACS Catalysis Lectureship for Advancement of Catalysis Science (2017)
- US EPA Presidential Green Chemistry Challenge Award (2016)
- Nankai-Asymchem Lecture (2016)
- Editor-in-Chief, Organometallics (2015)
- First Japanese Society of Coordination Chemistry Award for Creative Work (2015)
- Xingda Lecture, Peking University (2015)
- Closs Lecturer, University of Chicago (2014)
- Dalton Lecturer of the Royal Society of Chemistry (2011)
- Defense Science Study Group (2010-2011)
- Blavatnik Award, New York Academy of Sciences (2009)
- Arthur C. Cope Scholar Award, American Chemical Society (2009)
- Bessel Fellow of the Alexander von Humboldt Foundation (2008)
- Camille Dreyfus-Teacher Scholar (2006)
- Stephen and Margery Russell Distinguished Teaching Award (2005)
- Cottrell Scholar, Research Corporation (2004)
- David and Lucile Packard Fellow in Science and Engineering (2004)
- NSF CAREER Award (2003)
Selected Publications
Roque, J. B.; Shimozono, A. M.; Pabst, T. P.; Hierlmeier, G.; Peterson, P. O.; Chirik, P. J. “Kinetic and thermodynamic control of C(sp2)–H activation enable site-selective borylation.” Science 2023, 382, 1165-1170.
Hierlmeier, G. Tosatti, P.; Puentener, K.; Chirik, P. J. “Arene insertion with pincer-supported molybdenum-hydrides: Determination of site selectivity, reaction rates, and arene complex formation.” J. Am. Chem. Soc. 2023, 145, 21017-21039.
Mills, L. R.; Gygi, D.; Simmons, E.; Wisniewski, S.; Kim, J.; Chirik, P. J. “Mechanistic investigations of phenoxyimine cobalt(II)-catalyzed C(sp2)–C(sp3) Suzuki-Miyaura cross coupling.” J. Am. Chem. Soc. 2023, 145, 17029-17041.
Kim, S.; Kim, J.; Zhong, H.; Panetti, G. B.; Chirik, P. J. “Catalytic N–H bond formation promoted by a ruthenium hydride complex bearing a redox-active pyridine-imine ligand.” J. Am. Chem. Soc. 2022, 144, 20661-20667.
Mohadjer Beromi, M.; Younker, J. M.; Zhong, H., Pabst, T. P.; Chirik, P. J. “Catalyst design principles enabling intermolecular alkene-diene [2+2] cycloaddition and depolymerization reactions.” J. Am. Chem. Soc. 2021, 143, 17793-17805.
Park, Y.; Kim, S.; Tian, L.; Zhong, H.; Scholes, G. D.; Chirik, P. J. “Visible light enables catalytic formation of weak chemical bonds with molecular hydrogen.” Nat. Chem. 2021, https://doi.org/10.1038.
Mohadjer Beromi, M.; Kennedy, C. R.; Younker, J. M.; Carpenter, A. E.; Mattler, S. J.; Throckmorton, J. A.; Chirik, P. J. “Iron catalyzed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes.” Nature Chem. 2021, 13, 156-162.
Rummelt, S.; Peterson, P.; Zhong, H.; Chirik, P. J. “Oxidative addition of aryl and alkyl halides to a reduced iron pincer complex.” J. Am. Chem. Soc. 2021, 143, 5928-5936.
Pabst, T. P.; Quach, L.; MacMillan, K. T.; Chirik, P. J. “Mechanistic origins of regioselective in cobalt-catalyzed C(sp2)–H borylation of benzoate esters and arylboronate esters.” Chem 2021, 7, 237-254.
Joannou, M. V.; Hoyt, J. M.; Chirik, P. J. “Investigations into the mechanism of inter- and intramolecular iron-catalyzed [2+2] cycloaddition of alkenes.” J. Am. Chem. Soc. 2020, 142, 5314-5330.
Kennedy, C. R.; Zhong, H.; Macaulay, R.; Chirik, P. J. “Regio- and diastereoselective iron-catalyzed iron-catalyzed [4+4] cycloaddition of 1,3-dienes.” J. Am. Chem. Soc. 2019, 141, 8557-8573.
Related News

“I wrote a book!” Chemistry concentrators offer a few words on the senior thesis

EarthWeek Chemsplainer: former postdoc bio-derives building blocks for sustainability




