At Frick, Earth Day is in the Details
Suong Nguyen bought the first container of sawdust for her polymer chemistry research off of Etsy, the handmade craft website.
Later, she would find a more traditional route to access the Ponderosa pine, cedar, and larch she needed for her work. But at the time, Nguyen was eager to prove that a process the Knowles Lab has been refining for years, namely Proton-Coupled Electron Transfer (PCET), could provide a more environmentally sustainable, single-step method for breaking down carbon-carbon bonds in biomass at room temperature. Etsy provided a means to get started quickly, so that’s the route she used.
It is customary to talk about Earth Day in terms of global treaties and national policies. But for scientists, the significance often comes down to intricate choices made at the bench and then translating that work to the incremental, painstaking process of improving the planet’s health. As part of her own contribution to that goal, Nguyen started with the sawdust from which she would extract native lignin – a complex aromatic polymer that gives the cell walls in plants their rigidity and strength.

Photo courtesy of the Andlinger Center for Energy and the Environment.
Lignin accounts for roughly 30% of all organic carbon on the planet, so making good use of it is a compelling scientific pursuit. It is often mentioned as an environmentally sustainable alternative to petroleum products. But because of its complex structure, degrading lignin into its monomer is expensive and complex, requiring high temperatures and harsh conditions that don’t accommodate the criteria for sustainability.
Professor of Chemistry Rob Knowles and his lab, including Nguyen, thought it could be done under milder conditions. As it turns out, they were right.
Nguyen has since published the proof-of-concept paper for light-driven depolymerization of native lignin in ACS Catalysis. Today, she is applying that same PCET method to new materials, most recently synthetic plastic, adding to the quiver of sustainable tools that scientists can access in their investigations.
“We were just very excited about the idea of applying the chemistry we developed in our lab to help address a really cool and important problem in polymer chemistry,” said Nguyen, a fourth-year graduate student who was named the 2020-21 Maeder Fellow in Energy and the Environment. “That’s why we tried the chemistry with lignin first. We found that it worked quite well.
“In general,” said Nguyen, “we hope our studies will help to establish a general set of technologies for C–C bond cleavage chemistry in macromolecules and fundamentally impact the field of sustainable polymers.”
SCIENCE WITH SAWDUST
The goal was to investigate whether PCET and photoredox catalysis could serve as a viable method for converting biomass into small-molecule products in an Earth-friendly way, and whether it could do this more selectively than the harsher conditions typically employed.
“PCET reactions are a really interesting class of mechanisms where protons and electrons are exchanged together in a concerted process,” said Knowles. “Nature figured out how to do this really well: all of the biological redox processes that are important for life on earth, like photosynthesis, involve these coupled motions of protons and electrons.
“What our lab has been interested in doing is taking that framework from these biological systems and applying the lessons and principles to organic synthesis, particularly the ability of PCET to be able to form or break certain bonds that are difficult to break or form using conventional reagents.”
Graphic courtesy of the Knowles Lab.
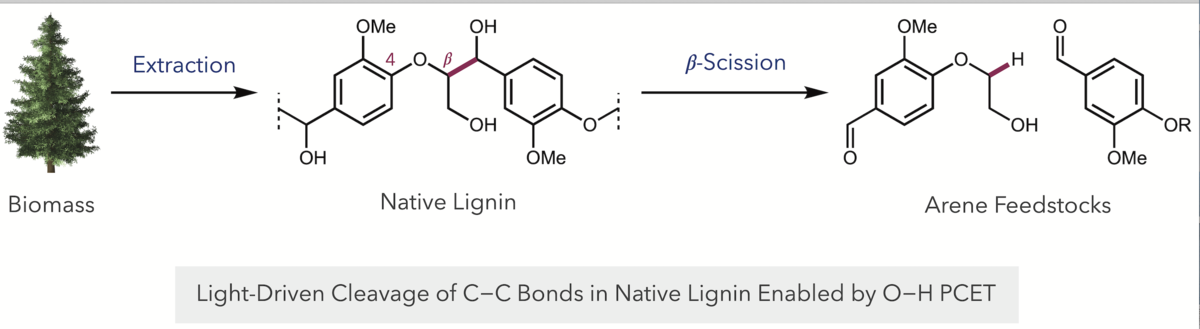
To apply this process to lignin, Nguyen first had to find a mild chemical reaction that would sever the bond between the lignin and the hemicellulose in order to extract lignin without significantly modifying its structure.
Next, Nguyen ran the depolymerization reaction using the PCET protocol. In the reaction “pot,” she placed the lignin powder and a photocatalyst (e.g. iridium complex), a weak base and a hydrogen atom transfer co-catalyst and shone a blue light on the reaction mixture at room temperature. This C-C bond cleavage reaction resulted in the generation of a well-defined mixture of small-molecule products, one of which is vanillin. Vanillin is used in the food industry to produce Vanilla extract. It was only a small quantity, Nguyen said, and so the chemistry is not likely to spur a new method for producing something you can use in your kitchen.
But it was a successful experiment, because the lab was just after a proof-of-concept.
“We wanted to show that the chemistry is viable,” said Nguyen. “We’re focused on how to use a much more selective, milder, efficient chemistry to form well-defined products that you can easily isolate from the reaction mixture,” said Nguyen. “And we proved that. We were able to isolate Vanillin cleanly.”
The concept held for further investigations with lignin from ponderosa, cedar, and larch.
Today, the lab is expanding the use of the PCET process to degrade other classes of macromolecules using the same techniques.
“We want to show the polymer community that, hey, this is a good method we developed and perhaps a milder and more selective way to address the problems you are working on,” said Nguyen. “We hope to introduce our chemistry to people in different fields.”
