DNA Barcoding Advances Programmable Nanoparticle Self-Assembly
More than two decades ago, scientists demonstrated that the self-assembly of nanoparticles – for use in fabricating miniaturized devices, for example – was possible if the nanoparticles could be labeled with a known number of DNA molecules.
Such a labeling technique would allow them to create well-defined nanocomposites simply by mixing DNA-tagged nanoparticles together. For this approach to work, scientists would need to precisely control the number of DNA molecules, or DNA valency, on the nanoparticle surface.
But the challenge to actually do that, in practice, went unmet for 25 years.
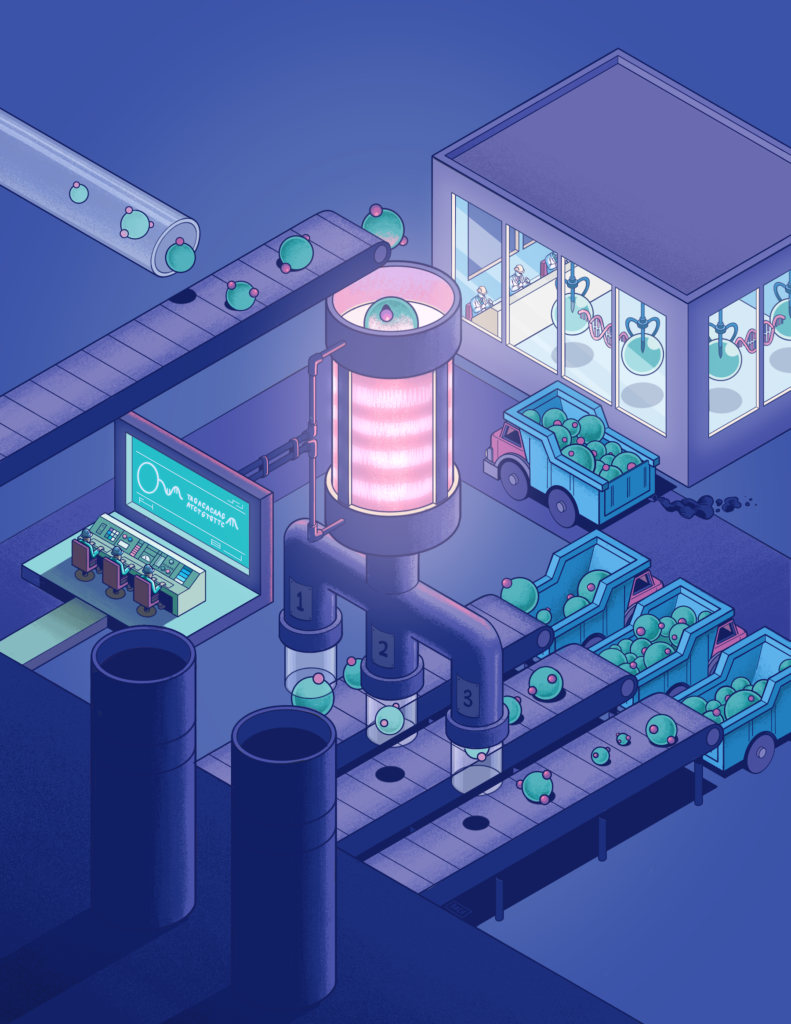
In this artist's rendition using a metaphorical factory, short DNA barcodes allow nanoparticles to be digitally sorted by the number of DNA molecules attached to them, regardless of their size.
This week, the Department of Chemistry’s Yang Lab publishes research on the first sorting technology that distinguishes nanoparticles through the mechanism of Watson-Crick digital recognition, thus enabling a new range of molecule-like building blocks.
The lab accomplished this feat by exploiting DNA’s programming language—its ability to selectively associate with and recognize complementary sequences—through a process called DNA barcoding.
DNA barcoding functions much like the codes used by supermarket scanners to identify a product. In this case, researchers tapped into the short genetic sequences in DNA as a means of sorting the nanoparticles to which they are attached.
The technique allows for the assembly of complex static and dynamic structures with predictable valency-defined nanoparticles.
The paper, Sorting Nanoparticles by Valency with DNA Barcoding, was published this week in the Journal of the American Chemical Society (JACS).
Using valency-defined nanoparticles to do novel nanochemistry wasn’t specifically the Yang Lab’s idea. “It’s a concept that’s been floating around in the literature for a long time. But it hasn’t been demonstrated,” said Nyssa Emerson, a postdoctoral associate and first author on the research paper, and the scientist whom Professor of Chemistry Haw Yang credits with the persistence to solve the challenge.
“What is conceptually new and interesting here is that we could use that programmable code to actually separate these nanoparticles out by the number of DNA molecules attached,” said Emerson. “We can change the letters of that code, and that tells us what DNA molecule will react with what other DNA molecule.
“There’s really not anything else that is this programmable and easily made.”
To demonstrate proof-of-concept, the researchers used nanocrystals of gold as the representative nanomaterial.
“The way we put the DNA on the nanoparticles, it just has these particular functional groups that like to interact with gold,” said Emerson. “Gold nanocrystals also have a lot of interesting optical properties. When we built these composite structures, they actually interact uniquely with light. So that was another reason to work with that material.”
How they did it
The challenge to create a sorting system can be generally framed as a problem of ratios—nanoparticle size to DNA molecule size. If you’re looking at a small nanoparticle, it’s easy to find the attached DNA—as if you were trying to tag a lemon sitting next to an orange.

Nyssa Emerson, a postdoc in the Haw Yang Lab and first author on the research.
Previous approaches to do this were effective for a narrow set of spherical nanomaterials, with diameters up to 30-40 nm. But that size limitation has stymied work in the field. Because for larger nanoparticles, the DNA molecule appears to recede, eventually becoming indistinguishable on the surface and thus useless as a means of distinguishing one nanoparticle from another.
The Yang Lab’s concept is implemented by barcoding the DNA molecules attached to a nanoparticle with a short, keyword sequence whose reverse complement is fixed to a solid support. The nanoparticle’s DNA valency then becomes “encoded” in its affinity, enabling the capture of like DNA sequences on the nanoparticles.
“Every nanoparticle will have this little barcode on it. And our column has another barcode that matches up with the one on the nanoparticle. And that’s how the separation works,” said Emerson. “Because that interaction is so specific, we’re able to see that really tiny DNA molecule on this giant nanoparticle. That selectivity and specificity for the complementary code is really strong. That’s the biology of it.”
Yasmine Eichbaum ‘19, a chemistry concentrator, completed her thesis work on this research.
“Haw’s lab is interested in developing novel single-molecule spectroscopy methods to better understand complex biological environments,” said Eichbaum, now a clinical research coordinator at the University of California, San Francisco.
“One aspect of this project was to design and characterize plasmonic nanostructures that could act as biosensors. By creating a robust separatory method, Nyssa provided the foundation for this research topic because this method allows one to design highly predictable structures with gold nanoparticles of various sizes.”
“Sorting Nanoparticles by Valency with DNA Barcoding” was authored by Nyssa Emerson and Haw Yang, and supported with funding from the Gordon and Betty Moore Foundation (grant #4741).
