In partnership with the NSF and the NIH: The Mo Lab and antibiotics discovery
One of the most interesting aspects about antibiotics is their origin story.
It makes sense to anthropomorphize a bit and think of them as stemming from bacterial turf wars in the soil and in the human body. Researchers like Princeton Chemistry Professor Mohammad Seyedsayamdost investigate these microbes for the chemical signals or toxins they use to repel other bacteria infringing on their territory.
In other words, if bacteria can successfully repel or even kill rivals with these molecules, then they can be wielded to fight human disease, too.
Seyedsayamdost’s partnerships with the National Science Foundation (NSF) and the National Institutes of Health (NIH) have led to remarkable discoveries in the field of antibiotics and infectious disease by tackling the global antimicrobial resistance crisis.
Examples within the past year alone include discoveries of potent and selective antibiotics against the agents of Melioidosis and C. diff infections. The therapies uncovered by the Mo Lab against both are now in the developmental pipeline.
“The NSF and the NIH have both been fantastic partners for us,” said Seyedsayamdost. “The research we’ve done and the discoveries we’ve made would not be possible without either of them.”
Lack of pharma funding
Pharmaceutical companies are not as involved in developing new antimicrobials because their priorities generally lie with drugs to treat cancer or neurodegeneration, for example. So the greatest source of funding for antibiotic development is federal agencies like the NSF and NIH working with academic labs, small biotech companies, and individual researchers.
Without them as funding sources, the whole field “sort of stalls,” said Seyedsayamdost.
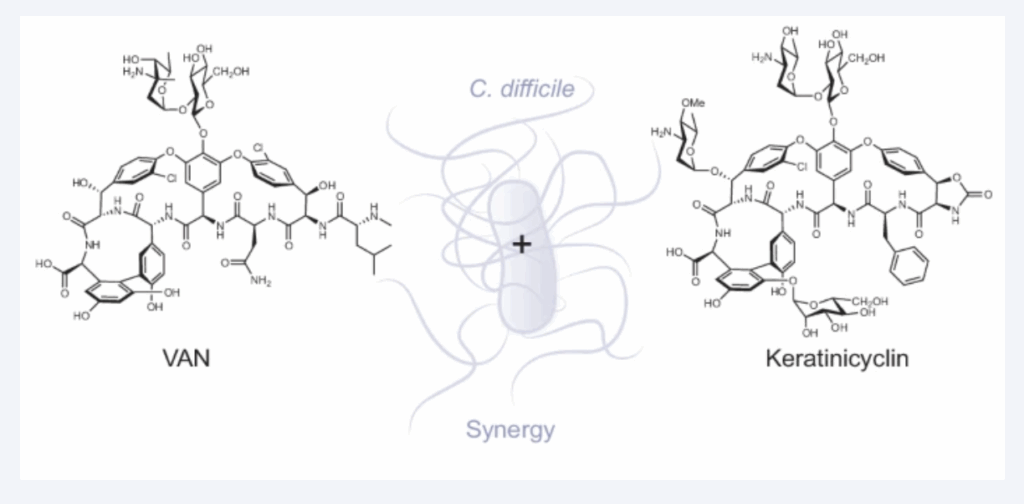
In research funded by the NIH, a recent paper in Nature Chemical Biology by the Mo Lab reports the mechanism of action of a recently discovered glycopeptide antibiotic keratinicyclin B, research that will in turn support the fight against C. Diff infections.
The Mo Lab has worked with the NSF and the NIH in antibiotics discovery, uncovering a vast range of possible new medicines through its HiTES technology, for which Seyedsayamdost won a MacArthur Fellowship. Introduced in 2014, HiTES, for High Throughput Elicitor Screening (HiTES), allows him to screen and identify the conditions that give rise to new chemical signals and antibiotics.
More recently, the lab is following a new direction in low-dose or subinhibitory antibiotic combinations. That promising new direction has received considerable support from the NIH.
The future of antibiotics: combination therapy
One of the challenges of current antibiotics is that they are broad-spectrum drugs. They work well but they kill broadly, taking down everything in their path; not just disease-causing pathogens but also “good” bacteria that the body needs to maintain health.
Under the Mo Lab’s combination therapy, researchers use a very small dose of one antibiotic—not enough to impact bacterial growth or to kill bacteria, but enough to alter metabolism and “open up” a new pathway that is now required for growth. Researchers then use a second molecule to effectively inhibit or block that pathway, thwarting the microbe’s ability to grow.
One of the great attributes of this approach is its specificity. Because combination therapy revolves around the metabolic idiosyncrasies of a specific pathogen, the treatment affects that microbe and that microbe alone, leaving the rest of the bacterial system—the good bacteria—unscathed. The low-dose combo allows for a balanced microbiome, accomplishing the challenging task of killing a difficult-to-treat pathogen while leaving much more susceptible, beneficial bacteria unharmed.
Benefitting the public
In addition to the research on Melioidosis and C. Diff, the Mo Lab has generated at least half a dozen antibiotic leads currently making their way through the developmental pipeline. This is good news for the public, since the development of new antibiotics has been alarmingly slow.
And because of its specificity in targeting dangerous pathogens only, low-dose combination therapy could be one answer to the growing, global resistance to broad-spectrum antibiotics that have been used indiscriminately for decades.
What do peer scientists think about low-dose combination therapy?
Pierre Stallforth, head of paleobiotechnology at Leibniz-HKI in Germany and internationally recognized for his work on bacterial signals: “It is a very creative and innovative strategy to antibiotic discovery.”
Steven Laken, biotechnology entrepreneur and CEO of Advise Connect Inspire LLC in Massachusetts: “There is currently a CDI pandemic in the U.S. and the combination therapy, as well as other leads discovered by the Mo Lab, are helping fill our antibiotics pipelines, which is desperately needed. The combination therapy is especially inventive.”
In partnership with the NSF and NIH
“The funds and grants that we receive from the NSF and NIH fulfill so many different purposes,” said Seyedsayamdost. “They fund so much of what academia does, what research does, and what ultimately benefits the entire country in terms of innovation, new drug leads, and training personnel who then go on to biotech and pharma companies where they contribute to new innovations and therapies. All of that starts with simple NIH and NSF funding at our labs.”
