Jacobs Group offers first principles approach to predicting behaviors of nonequilibrium systems

Scientists investigating phase-separated mixtures at thermal equilibrium use well-developed, broadly accepted theoretical tools to predict how these systems will behave.
But what about living cells that are driven out of equilibrium by fuel consumption, as with the common example of the ATP-consuming enzymes? Under these circumstances, the predictive capacity shrinks because we don’t have a clear understanding of exactly what happens away from thermal equilibrium.
Can scientists rely on the same, established tools used for thermal equilibrium, or is a new approach needed?
The Jacobs Group answers with a paper that lays down a first-principles approach for modeling and predicting the behaviors of non-equilibrium mixtures. Their work underscores the central role that interfacial properties have in governing non-equilibrium phase separation, and emphasizes that all length scales must be considered when modeling these systems.
PAPER: “Interfacial Effects Determine Non-Equilibrium Phase Behaviors in Chemically Driven Fluids”
JOURNAL: Proceedings of the National Academy of Sciences (PNAS)
AUTHORS: Graduate Student Yongick Cho; and Assistant Professor of Chemistry William Jacobs.
WHAT IT IS: The Jacobs Group proposes a nonequilibrium theory of phase coexistence that connects a microscopic description of the interface to a mesoscopic model of the bulk phases. Researchers show that, when describing biomolecular condensates that are continuously driven out of equilibrium by chemical reactions, it’s necessary to consider all length scales, from the microscopic to the macroscopic, from individual molecules to the collective.
Their approach has broad implications for our understanding of the stability and nucleation kinetics of biomolecular condensates.
WHAT THEY DID: Researchers set out to answer one driving question: is there a need to account for reactions happening at microscopic scales in order to predict organization and assembly at macroscopic scales? Answer: yes, resoundingly. Existing tools do not make accurate predictions in all parameter regimes.
So the group pursued theoretical descriptions and simulations of non-equilibrium, phase-separating systems. In their investigations, they prevented the system from relaxing to thermal equilibrium by continuously supplying a chemical fuel, mimicking the steady supply of ATP in living cells. They were able to demonstrate through simulations that mesoscopic fluxes in these systems are dependent on non-equilibrium microscopic fluctuations at the interface between coexisting phases.
The simulations guided the development of a first-principles theory to predict phase behaviors in systems driven far from equilibrium by chemical reactions. In particular, their theory predicts coexistence curves, the spatial localization of mesoscopic fluxes near phase-separated interfaces, and droplet size-scaling relations.
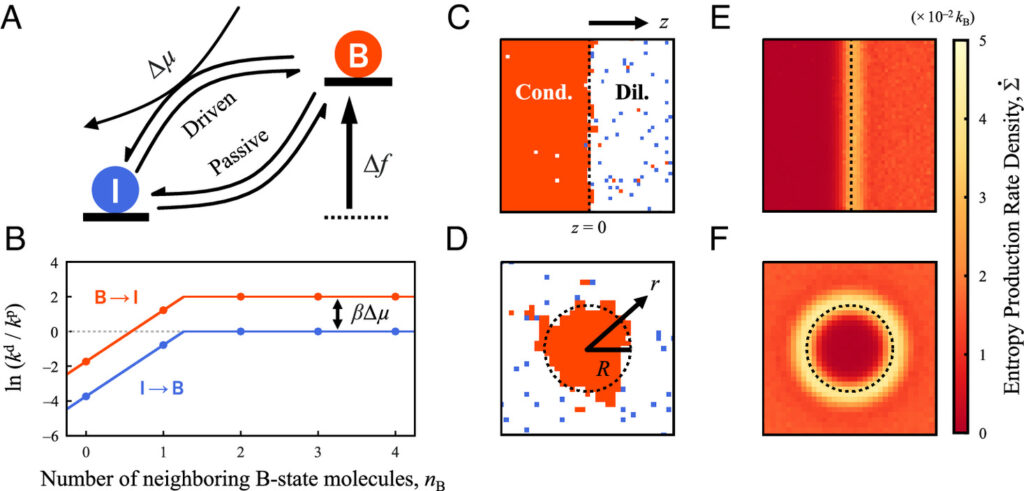
A molecular-scale model of nonequilibrium phase coexistence in chemically driven fluids.
COMMENT FROM WILL JACOBS, ASSISTANT PROFESSOR OF CHEMISTRY: “The main motivation is to understand the spatial organization of macromolecules inside of living cells, and biomolecular condensates are one important aspect of that. All the various molecular components inside of the cell are not homogeneously distributed. There is a lot of fine-tuned spatial organization, and it’s really important to understand the principles that govern that.
“In this case, we focused on chemically driven fluids in non-equilibrium systems. What we show in this paper is that truly at every scale, from the microscopic up to these larger length scales, there are clear signatures of nonequilibrium effects. You really cannot assume an equilibrium thermodynamics approach at any of those scales.
“What’s important here is that we have a new way of being able to predict the phase behavior of nonequilibrium phase-separated droplets from first principles, meaning that we start with what happens on the microscopic scale and then predict macroscopic behaviors. That’s really the key insight.”
COMMENT FROM YONGICK CHO, FIRST AUTHOR: “This work reveals that the novel behaviors of droplets in biological systems stem from the droplet interface, which was not fully accounted for in previous theories. Even though the interface is a thin layer whose volume is negligible, it possesses a distinct chemistry from the rest of the system by being situated between the droplet interior and exterior.
“Therefore, chemical reactions taking place on the interface affect the droplet in a way that cannot be attributed to either the interior or exterior, leading to the behaviors that were not fully understood.
“In order to reveal the role of the interface, we had to employ a simulation technique that is able to capture the fluctuations of the interface. This is achieved by simulating each molecule as a coarse-grained particle so that the molecular-scale effects concentrated near the interface can be correctly captured.”
FUNDING SOURCE: This research is supported by the National Science Foundation (DMR-2143670).
