Mo Lab Elucidates the Mechanism of a Metalloenzyme Superfamily

The Department of Chemistry’s Mo lab has elucidated the mechanism of carbon-carbon bond formation catalyzed by a superfamily of metalloenzymes during the biosynthesis of a bacterial natural product.
Introduction of carbon-carbon bonds at unactivated centers is a “holy grail” in chemistry. Metalloenzymes have long served as models for such reactions, revealing nature’s approach to these transformations. Studying the largest-known family of enzymes in nature – the radical S-adenosylmethionine or rSAM enzymes – the lab trapped and characterized a never-before observed, short-lived radical intermediate during installation of a lysine-tryptophan carbon-carbon linkage introduced by the rSAM enzyme SuiB.
In collaboration with the Britt lab at the University of California, Davis, the team utilized electron paramagnetic resonance spectroscopy in conjunction with isotope labeling, kinetics, and computation to catch glimpses of the unique lysine-crosslinked tryptophan radical. The radical carried a highly unusual tetrahedral center – the first of its kind.
“We started off drawing this species on the board several years ago, postulating that it existed based on biochemical experiments we had performed,” said Alessio Caruso, co-lead author on the paper and a fifth-year graduate student in the Mo lab. “So, you have it on paper, you think about what could be going on, you do experiments to test it, you scrutinize it. But then to actually catch it in the act was the best evidence we could possibly ask for.”
The paper, “Trapping a Crosslinked Lysine-Trypotophan Radical in the Catalytic Cycle of the Radical SAM Enzyme SuiB,” was published last month in the Proceedings of the National Academy of Sciences (PNAS).
Understanding these intermediates is an important step in gaining insights into how enzymes catalyze reactions in nature. The Mo lab has done considerable research on new types of reactions carried out by these highly versatile enzymes, and began publishing papers on this superfamily in 2015.
SUPER FAMILY
The rSAM superfamily comprises some 570,000 member enzymes. Using an iron-sulfur cluster and cofactor SAM, members of this superfamily typically generate a reactive 5’-deoxyadenosyl radical, which initiates the radical reaction. About one-quarter of the family has extra iron-sulfur clusters. Although the presence of these “auxiliary” clusters was known, their precise function was not understood. Their plenitude opens the door to broader questions: namely, what are they doing in a quarter of the enzymes in this superfamily?
“Our results reveal the mechanism with which rSAM enzymes generate carbon-carbon bonds,” said Caruso. “They further reveal the function of the auxiliary iron-sulfur cluster as an oxidant of the unusual lysine-tryptophan radical. The fact that this pathway may be widely employed is exciting. It’s telling you that nature, the most efficient chemist, is using this strategy all over the place.
“And these enzymes are widespread – they’re found in insects and bacteria and people, catalyzing reactions that are essential, for example, in making DNA from RNA or, in this case, the production of an antibiotic.”
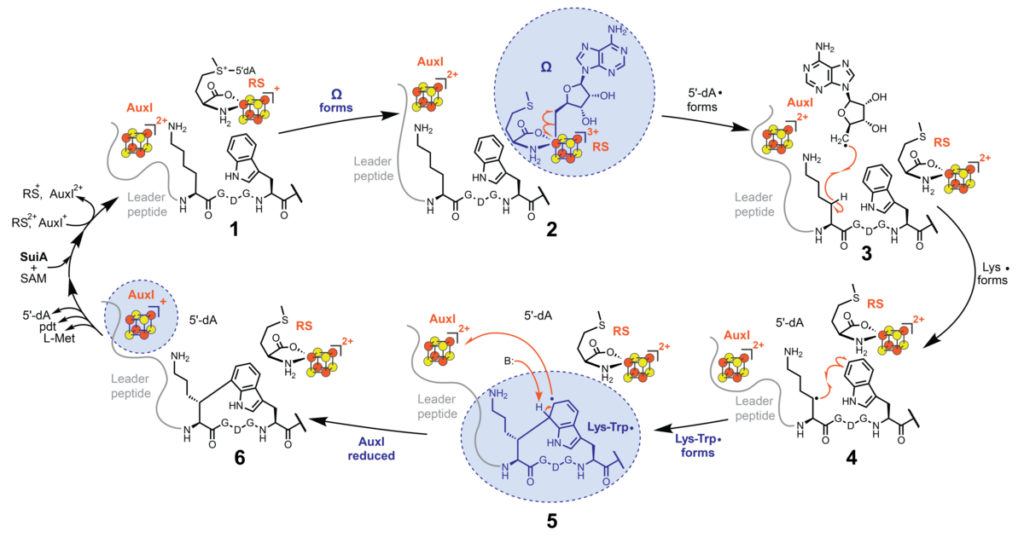
The Mo lab reports on the catalytic cycle of the carbon-carbon bond forming radical SAM enzyme SuiB, revealing an unprecedented lysine cross-linked tryptophan radical intermediate.
Researchers were able to trap the radical by plunging the sample into liquid nitrogen, which essentially freezes the reaction at a particular point in the catalytic cycle so that intermediates can be observed.
“The unusual tetrahedral shape in the radical is a result of making that radical by effectively adding an electron to the tryptophan,” said Mohammad Seyedsayamdost, professor in the Department of Chemistry. “Thus far, all observed tryptophan radicals have resulted from removing an electron from tryptophan.
Caruso added, “By adding an electron – and making a new bond in the process – the tryptophan radical that is generated becomes very reactive. The enzyme then controls its reactivity by guiding its reaction with the auxiliary iron-sulfur cluster at the right time, thereby re-establishing tryptophan aromaticity and completing product formation.”
This work was funded through an NSF CAREER Award (No. 1847932); the Edward C. Taylor-Eli Lilly Fellowship in Chemistry, an NIH R35 Grant (No. 1R35GM126961-01); an NSF XSEDE Grant (No. CHE-030089); and an NSERC Postdoctoral Fellowship.
