Mo Lab takes aim at C. diff pathogen through antibiotic synergy
Antibiotic resistance is a well-documented healthcare crisis. The problem is multi-faceted and includes issues such as the cycle of treatment-and-relapse that results when antibiotics weaken or destroy an organism’s healthy bacteria, leaving the system vulnerable to new infections.
This is the case with Clostridioides difficile (C. diff), an opportunistic pathogen that is responsible for over 12K deaths each year in the U.S. alone with associated healthcare costs of $1 billion annually.
C. diff becomes a runaway problem in the human gut when the very antibiotic approved to treat it lays waste to beneficial gut bacteria, thereby setting the stage for returning infections.
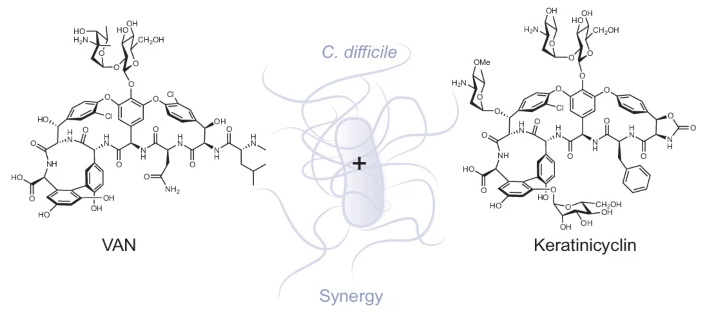
The Mo Lab's new paper reports the mechanism of action of a recently discovered glycopeptide antibiotic keratinicyclin B.
“A C. diff infection typically occurs after antibiotic use, making it somewhat of a manmade condition. It’s very paradoxical,” said Vasiliki Chioti, lead co-author of a recent paper from the Seyedsayamdost Lab as part of their mission to uncover new antibiotics. “You treat the infection, but then the patient relapses, necessitating further treatment. This recurrent cycle can lead to high mortality rates, especially among older adults and immunocompromised individuals.”
Now, the lab reports the discovery of a potent combination of two antibiotics that could provide a solution to both the infection and its recurrence.
Their discovery of a synergistic relationship between keratinicyclin B (KCB) and the old standard vancomycin reveals an effective treatment at a much lower concentration of each, so there is little, if any, collateral damage to healthy bacteria. In addition, KCB has a different mode of action in inhibiting bacterial growth compared to vancomycin: it interferes with peptidoglycan degradation.
The research, Potent and specific antibiotic combination therapy against Clostridioides difficile, was published last month in Nature Chemical Biology in collaboration with Molecular Biology and researchers at Emory University.
“The combination is remarkably selective against C. diff,” said Professor of Chemistry Mohammad Seyedsayamdost. “We tested a panel of commensal and beneficial bacteria in the human microbiome, and none were affected by the combination, whereas C. diff was completely wiped out. “
A Novel Antibiotic Identified by the Mo Lab
The polymer called peptidoglycan forms an important structural network that strengthens bacterial cell walls with layers of glycan strands. These strands are crosslinked to form a robust network that protect C. diff cells.
Vancomycin, the only naturally occurring glycopeptide approved by the F.D.A. to treat C. diff, works by essentially blocking peptidoglycan crosslinking. But it also kills surrounding bacteria, wiping out even those that are necessary to gut health. The C. diff pathogen reinfects the host and the cycle repeats.
Enter KCB, a novel antibiotic the Mo Lab identified several years ago through its groundbreaking High Throughput Elicitor Screening (HiTES) technology. Seyedsayamdost received a 2020 MacArthur Award for the use of HiTES in the discovery of new antibacterial agents and other potentially therapeutic molecules from natural sources.

Vasiliki Chioti, who defended her doctorate in May, is co-lead author of the paper, published last month in Nature Chemical Biology.
“Among the many experiments, we observed C. diff cells by microscopy following treatment with the antibiotic KCB,” Chioti said. “While vancomycin inhibits peptidoglycan biosynthesis, we found that KCB inhibits the degradation of the peptidoglycan polymer. Although both antibiotics disrupt peptidoglycan metabolism, vancomycin targets the anabolic pathway, while KCB interferes with the catabolic pathway.
“The synergistic interactions between the two antibiotics leads to cumulative effects on the growth of C. diff. Synergy is typically observed when two antibiotics inhibit different steps in the same pathway or two different pathways altogether. In this case, it’s two steps in the same pathway.”
The lab began investigating KCB several years ago when a former postdoc, Fei Xu, first isolated the compound. Researchers noted then that while it belonged to the same class of molecules as vancomycin, it responded to C. diff with a much narrower spectrum of activity. It was not a broad-spectrum antibiotic. Rather, it was selective against C. diff.
Computational analyses performed by collaborators at Emory University allowed the team to visualize intermolecular interactions that helped corroborate the findings.
“The cool thing about the modeling is, we know that vancomycin binds the precursor through five hydrogen bonds,” said Chioti “And the same H-bonding partners that vancomycin uses to bind the peptidoglycan are repurposed by KCB to bind its target. That means that this framework of hydrogen-bonding partners is a conserved prevalent scaffold used to bind multiple molecular targets.”
At this point, the lab has completed only in vitro studies. The next step would involve in vivo work using live mouse models infected with C. diff and then treated with the KCB/vancomycin approach as a drug candidate.
“Finding novel antibiotics that work in vitro with a new mechanism of action is difficult to do and therefore a great start,” said Seyedsayamdost. “But the ultimate goal is to develop these toward medical application and enrich our repertoire of clinical antibiotics. That is the next step in this project.”
Adds Chioti: “We need to make sure that it works in the body, in the mammalian system. But it looks good so far.”
Authors on this paper are Vasiliki Chioti, Kirklin McWhorter, Tamra Blue, Yuchen Li, Fei Xu, Philip Jeffrey, Katherine Davis, and Mohammad Seyedsayamdost. This research was supported by funding from the National Institutes of Health (grant nos. 1R35GM147557 to K.M.D. and 1R01GM129496 to M.R.S.).
