Muir Lab Introduces a Groundbreaking Method for Protein Editing
The Muir Lab delivers a groundbreaking protein editing technology that, for the first time, enables the insertion of a lab-engineered cargo of modifications directly into the folded protein of living cells.
Protein modifications work deep inside the cellular microenvironment to influence an elaborate network of functions. Understanding and directing these modifications could give scientists a new tool to combat disease in the human body.
The lab’s striking technology allows researchers to replace tiny sequences inside cellular proteins, shaping the intricate “clockwork” of the very biomolecules that control human organ and tissue function, immunity, and disease states.
Their method works without disrupting the structure of the protein, another goal that has not been previously achieved.
Selective editing of this nature has already been achieved for DNA and RNA, its mechanism popularized through the CRISPR technology introduced to the world in 2012. But this editing technique has not been available for proteins. Scientists describe proteins as notoriously difficult to edit, mostly because they have a complex 3D folding mechanism.
The Muir Lab changes all that with a method named “protein transposition” that accomplishes insertion within a short, coordinated timeframe.
And because it does so when the protein is already folded, the method allows scientists to vault over a number of complicated steps that have foiled progress in the past.
A key stroke in their success is the use of split inteins—proactive protein domains within cells that can maneuver according to task—to conduct the cut-and-paste splicing of the tiny cargo of modifications. The Muir Lab has been studying and engineering split inteins for almost two decades now.

The authors of the Science paper: (Standing left to right) Postdoc Nicholas Tay and P.I. Tom Muir; and graduate student Yi Hua (seated).
The research was announced last month in a paper in Science: Protein Editing using a Coordinated Transposition Reaction. More recently, it was highlighted in separate commentaries in both Science and Nature.
“We knew that split inteins had the potential to do this, but it took us years to understand how these ‘Houdini’ proteins actually work, and then from this to engineer them for the desired protein transposition reactions,” said Tom Muir, the Van Zandt Jr. Class of ’65 Professor of Chemistry and principal investigator on the work.
“Put simply, this allows us to do chemistry on folded proteins and protein complexes, systems that were previously off the table for us and that constitute the vast majority of targets people would be interested in studying and manipulating using chemistry. We believe this is an important advance that will have utility in both the basic and applied biomedical sciences.”
Yi Hua, a graduate student with the Muir Lab and lead co-author on the paper, called the method a “huge advantage.”
“We will be able to access proteins that are extremely difficult to fold and also proteins that are extremely large, or localized in a large complex or on the cell membrane. All those proteins in the past that were extremely difficult to deal with should now be accessible with way less difficulty.”
In comments published on May 1, Science editors noted: “Such direct protein editing creates possibilities for introducing previously unachievable protein functions and their spatiotemporal controls at the cellular level.”
Drawing from DNA transposition
The Muir team drew their inspiration from a well-studied biological phenomenon that happens on DNA called transposition. Certain cellular components are charged with moving discrete DNA segments within the genome to other locations to alter the genetic material. The Muir team came up with the idea to apply this naturally occurring phenomenon to their own established work with protein function in general and with inteins in particular.
Inteins are polypeptide sequences that can excise themselves from the protein in which they are embedded and then reassemble within the same region. To translate this reactivity to bioengineering applications, the Muir lab used split inteins, which have a strong inclination to “find each other” while being attached to other proteins.
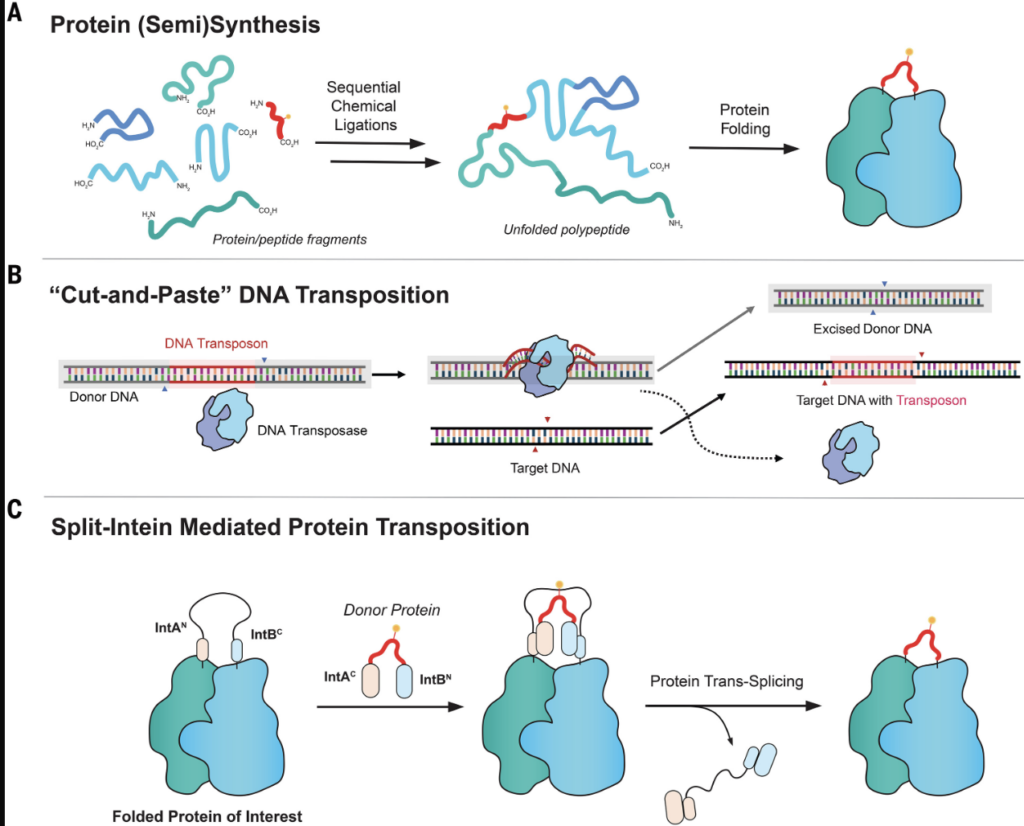
The design of a split intein-mediated protein transposition reaction. The approach circumvents the need to generate and manipulate protein fragments and does not require a folding step.
Researchers exploited that inclination to affect the insertion, or splicing of the modification cargo, on demand. They used pairs of engineered split inteins with tuned and balanced properties, putting one set of the partners onto the cargo and the other set within the protein itself. By tuning the design of these partners, they were able to make them splice together on a coordinated timescale in a way that maintains the fidelity of the protein fold.
“In Tom’s lab, we engineer inteins, and we have been doing that for a long time,” said Nicholas Tay, a postdoc in the Muir Lab and co-lead author on this research. “We engineer naturally split inteins to have higher association and faster splicing rates. Once they find each other, the proteins will undergo a natural folding process and that is what enables the chemistry necessary to splice together the two ends of the polypeptides they’re attached to.”
Researchers demonstrate the utility of this method using protein systems like the chromatin remodeling complex ACF, showing that protein transposition can occur both in vitro and within more complex biological setting likes the cell nucleus, which opens up a range of in situ applications.
The method can serve as a new tool to probe disease states in mammalian cells.
“In disease states, proteins can be turned on and off, or they can be turned on for an extended period of time,” said Tay. “Is it a post-translational modification that is causing this? Is it a specific mutation? How do we study this?
“The only way is by developing new tools to put in specific site locations of disease-modifying groups so that we can study what happens inside the cell at any given time. That’s what we’re doing here.”
The Muir Lab anticipates that large, public data sets on protein structure will enable the biochemical community of scientists to thoroughly investigate their novel methodology. Lab members said there has been an enthusiastic reception so far to the research.
With his customary reserve, Muir concluded: “As with any new technology time will tell. The best measure will be to see how broadly it becomes adopted and the best way to ensure that is for my lab to continue to use the approach to address important biology problems.”
This work was funded by NIH-GMS grant R01 GM086868 and NIH-NCI grant R01 CA240768.
