Photodriven Weak Bond Formation from Chirik Lab

As a springboard to understanding the significance of the Chirik lab’s latest research in sustainability, take the Haber-Bosch process.
Arguably the most important industrial advancement of the 20th century, Haber-Bosch ammonia synthesis conquered food scarcity by creating the means to mass produce fertilizer – fertilizer then used to fortify food harvests around the world.
But the production of ammonia – the building block for ammonium nitrate fertilizer – generates a problematic byproduct down the line: carbon dioxide. Lots of it. More than two tons of carbon for every ton of fertilizer. It accounts for an estimated 1.4% of global carbon dioxide emissions. So, while the process countered mass starvation, it also began ratcheting up the planet’s burden of greenhouse gases.
One of the main goals before chemists today is de-coupling food production from carbon. In part, this means finding a way to produce fertilizer through carbon-free ammonia synthesis: can it be done without Haber-Bosch?
The Chirik lab takes an important step towards this possibility with a fundamentally unique approach to the synthesis of chemical bonds. Researchers use visible light to drive the formation of weak element-hydrogen bonds, which lie at the heart of the challenge because they are so difficult to make.
The lab’s proof-of-concept paper, published this month in Nature Chemistry, lays out a simple method that involves shining blue light on an iridium catalyst to enable the formation of weak bonds at or near thermodynamic potential – that is, with no massive outlays of energy – without yielding a carbon byproduct.
“The big breakthrough here is being able to take light and then promote a chemical reaction to make a bond that’s really weak that you couldn’t do without an external stimulus,” said Paul Chirik, the Edwards S. Sanford Professor in Chemistry. “In the past, that stimulus has been coupled with making waste or consumption of electricity. Here, we’re doing it with light.
“We have this world of metal catalysts that have done amazing things – they’ve made ammonia, they’ve made drugs, they’ve made polymers. Now, we can do even more with them when we start looking at what happens when these catalysts absorb light,” he added. “So, you’re taking something that did really cool chemistry before and you’re juicing it with another 50 kilocalories.
“A whole world opens up. Suddenly, there’s a new class of reactions we can think about doing. Maybe distributive ammonia synthesis is one of them.”
SHINE A LIGHT
E-H bonds are simply a way of denoting any bonds you might make between hydrogen and another element. E-H bond strengths are highly dependent on the chemical structure of each element, but many of these bonds are weak – unstable and inclined to break easily and form hydrogen (H2). Most chemical reactions are driven by the formation of strong bonds as energy is released when more stable products are formed. It is the assembly of weak bonds that poses the challenge.
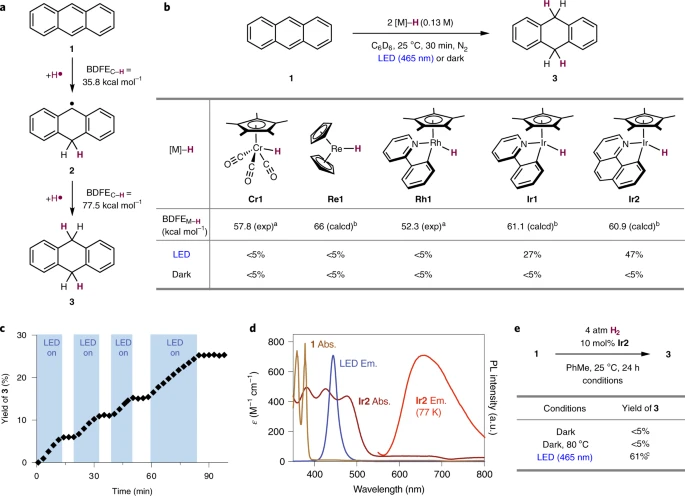
Proof-of-concept for photoinduced catalytic proton-coupled electron transfer (PCET) using H2.
The Chirik lab has found a way to make a weak bond by shining light on a catalyst; in this case, iridium.
This is how it works. Researchers chose a representative organic molecule, anthracene, which acts as a kind of platform on which the chemistry takes place inside the reaction flask. Shining blue light on iridium inside the flask gets it “excited,” meaning it has energy to drive the reaction. In this state, it bumps into the anthracene molecule and transfers a hydrogen atom to make a weak bond. The iridium catalyst then activates hydrogen gas, completing the cycle.
Utilizing hydrogen gas instead of carbon-based hydrogen sources – widely utilized in organic synthesis in the past – potentially provides a sustainable way to make weak chemical bonds without generating carbon byproduct.
Yoonsu Park, a postdoc in the Chirik lab and lead author on the paper, and Sangmin Kim, a 2021 Ph.D. graduate of the lab, came up with the idea of using photochemistry by reviewing weak bonds that appear in other reactions and extrapolating their lessons. Two additional authors on the paper, Greg Scholes, the William S. Tod Professor of Chemistry, and his graduate student Lei Tian of the Scholes lab, contributed insights into the role of blue light using a variety of laser experiments.
Park also determined which metal catalyst in the vast expanse of the periodic table would be the most effective in carrying out the reaction. Jumping off from previous lab work done with rhodium – another rare, expensive metal catalyst – he quickly zeroed in on iridium.
“This research shows that these kinds of transformations with hydrogen are actually possible to make a weak chemical bond without a byproduct,” said Park. “As a proof of concept, we take a much more operationally convenient molecule of anthracene and by using hydrogen reduce it to dihydroanthracene. In the process we are making very weak C–H bonds that are below potential to produce hydrogen.”

The reaction setup with a blue light emitting diodide (LED).
The Chirik lab is completing further investigations with this research as they advance the science to synthesize carbon-free ammonia. While scientists are not yet ready to jettison Haber-Bosch, the Chirik lab’s proof-of-concept is an important early step.
“We haven’t made ammonia yet catalytically. We have a long way to go on that goal,” said Chirik. “But it’s this idea of learning how to make these weak bonds that is so important and having important leads in this direction.
“The thing I like about this research is, it’s different. It’s fundamental chemistry, as basic as you can get. Nobody’s opening a plant on this research tomorrow. But we’re really excited about the concept, and we really hope that other people do this chemistry in other contexts.”
“Visible light enables catalytic formation of weak chemical bonds with molecular hydrogen,” by Yoonsu Park, Sangmin Kim, Lei Tian, Hongyu Zhong, Gregory Scholes, and Paul Chirik in the Department of Chemistry, Princeton University, appeared in the July 12, 2021 issue of Nature Chemistry, (https://doi.org/10.1038/s41557-021-00732-z). This research was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Catalysis Science Program, (Award DE-SC0006498), and the Andlinger Center for Energy and the Environment at Princeton University.
