Researchers unlock coveted bond connection
Researchers at Princeton University have introduced a long-awaited reaction capable of forming sp3-sp3 bonds whose presence increases a molecule’s complexity and its chances for clinical success as a drug candidate.
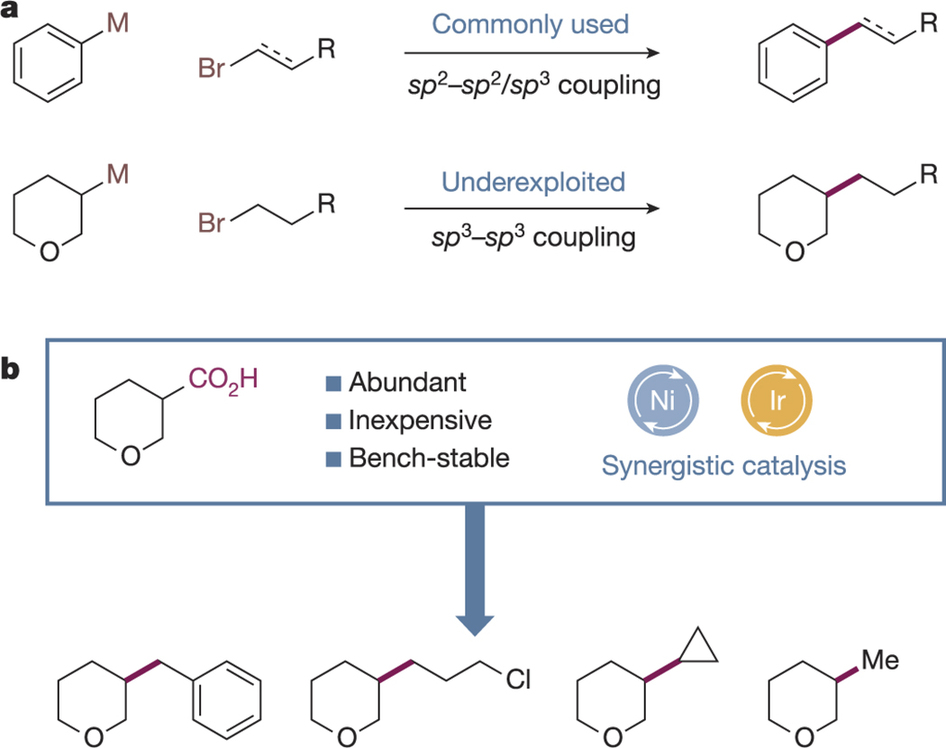
Published in Nature, the study detailed a mild and general method to couple sp3 carbon atoms – carbon centers defined by the fact that only single bonds connect them to their neighbors. Until now, this coveted reaction had resisted chemists’ efforts, even eluding transition metal catalysis, a powerful field that has enabled a staggering range of coupling reactions over the past 50 years.
“The reaction is a very unique way of approaching how you would join molecules together, and broadly expands the types of carbons you can connect,” said David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry and corresponding author on the work.
Their method revolves around the cooperation of two catalysts, a light-activated iridium catalyst and a nickel-based catalyst. Coined metallaphotoredox catalysis, this process circumvents roadblocks, such as undesired side reactions and an inability to form key intermediates, which had plagued other transition metal mediated attempts. Also, in contrast to previous, specialized versions of the reaction, the researchers’ strategy doesn’t require high temperatures, harsh basic compounds or additional zinc-based molecules.
The reaction succeeds by enlisting the two catalysts to bring together the molecules forming either side of the final sp3-sp3 bond. The light-activated iridium catalyst converts the commercially available starting compound known as a carboxylic acid into a ready partner. This intermediate is intercepted by the nickel catalyst, which can then incorporate the other chemical partner, called an alkyl halide. Finally, the nickel catalyst excises itself from the compound, releasing the desired product and resetting the cycle.
The team demonstrated the reaction’s generality as it proceeded smoothly with array of structurally diverse partners. Using this method, they also constructed the antiplatelet drug tirofiban in two steps from simple starting materials using their sp3-sp3 coupling reaction and another metallaphotoredox method recently developed in their lab. This example showcased the utility of their program for drug discovery though it holds potential for other industries as well.
“That’s what we really care about – inventing reactions that people will use,” MacMillan said. “We really want to do things that are enabling to people all around the world who care about making molecules.”
Read the full article here:
Johnston, C. P.; Smith, R. T.; Allmendinger, S.; MacMillan, D. W. C. “Metallophotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides.” Nature 2016, 536, 332.
