Scholes/Knowles Intro Method to Slow Charge Recombination
Drawing on inspiration from photosynthesis and the way it achieves high efficiency in plants, researchers in the Scholes and Knowles labs together with colleagues at Arizona State University introduce a bioinspired catalyst that lengthens the productive state of some chemical reactions.
Researchers employed proton-coupled electron transfer (PCET) to manipulate a photocatalyst in order to slow charge recombination, essentially mimicking the process that keeps photosynthesis churning along.
Their mechanism stalls one elementary step in the process by an order of magnitude relative to a reference compound. PCET is a class of reactions involving the transfer of an electron and a proton.
At the core of this collaborative research is a chemical model, a PCET substructure, developed by researchers at Arizona State. The model is linked with an iridium complex that effectively lessens the driving force of charge recombination, thus prolonging the active state in which chemistry can take place.
“The model is based on the oxygen-evolving complex in photosynthesis that is in every plant, the enzyme that makes oxygen for the whole world,” said Hannah Sayre, a former postdoc in the Scholes Lab and lead co-author on the study. “There are two amino acids nearby the oxygen evolving complex – hydrogen-bonded tyrosine-histidine – and these two amino acids shuttle electrons away from the oxygen-evolving complex, by a PCET mechanism, so that the enzyme is able to produce oxygen.
“Our collaborators have made this model, benzimidazole-phenol (BIP), that is chemically very similar to those two amino acids in photosynthesis. And so, we can use it to control the direction of electron transfer in catalysis.”
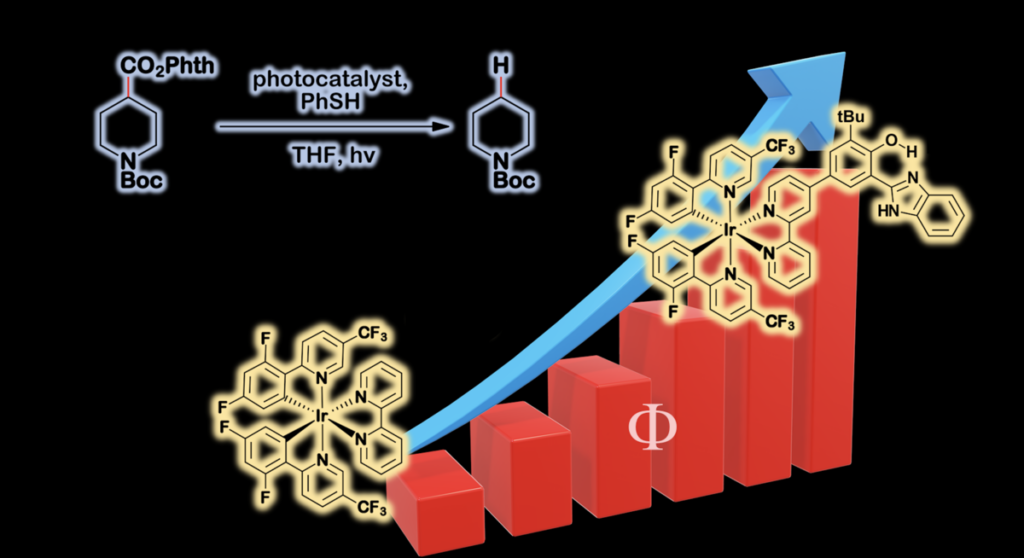
This graphic shows the quantum yield of the reaction, which is how researchers measure the efficiency of a light-activated reaction.
The paper, “PCET-based Ligand Limits Charge Recombination with an Ir(III) Photoredox Catalyst,” was published last week in the Journal of the American Chemical Society (JACS).
The research combines contributions to the mechanisms of photocatalysis from the Knowles lab; ultra-fast time resolved spectroscopy from the Scholes Lab; and the BIP model from Thomas and Ana Moore at the School of Molecular Sciences, Arizona State University.
ENERGY FRONTIER RESEARCH CENTER
The research fulfills one of the top priorities for the Princeton-based Bioinspired Light-Escalated Chemistry (BioLEC) group, an Energy Frontier Research Center established in 2018 and funded by the Department of Energy. BioLEC is directed by Gregory Scholes, Princeton’s William S. Todd Professor of Chemistry and chair of the department.
“This absolutely came about because of the Center. Rob, Tom, and Ana were the intellectual drivers of this idea,” said Scholes. “Here, we added an additional ‘circuit’ to the molecular photocatalyst that suppressed the deactivation pathway. Our circuit serves as a kind of buffer, holding the high energy species in place until the catalyst can initiate the chemical reaction. I think it’s a great success story.”
The Moores added, “Working with Rob and Greg and the Center students and postdocs has proven to be even more exciting and rewarding than we hoped for when the idea was first formulated for the BioLEC proposal.”
In nature, photosynthesis occurs when a plant absorbs light that generates a charge separation in its “reaction center.” That reaction drives both the oxidation of water and the fixation of carbon dioxide into fuels used by the plant. Charge recombination essentially short circuits the photosynthetic process. So, nature has evolved ways to sustain that separation using so-called redox relays.
Researchers used these very redox relays in their investigation as the essential components of a series of short-distance, fast redox-equivalent transfer steps that effectively compete against charge recombination.

Hunter Ripberger, a former graduate student in the Knowles lab and co-lead author on the paper.
“Evolutionary processes selected these redox relays where through the transfer of an electron and a proton between two hydrogen-bonded amino acids, it can do really fast transfers that further separate the charges,” said Hunter Ripberger, a former graduate student in the Knowles lab and co-lead author on the study.
“By moving the charges further apart quickly, you prevent that charge recombination. That leads to the efficiency of photosynthesis. We made use of that idea.”
The structure of the BIP model developed by the Moores is similar to those two amino acids.
“Sure enough, studies in the Scholes’ lab of the reengineered catalyst with the BIP showed longer lifetime for the reactive radical intermediate and – here’s the proof in the pudding – the yield of the reaction was more than doubled,” said Tom Moore. “Nature uses PCET in catalysis, and Mother Nature knows a thing or two about efficient, sustainable chemistry.”
Read the full paper in JACS here: https://pubs.acs.org/doi/10.1021/jacs.1c01701.
This work was supported as part of BioLEC, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award #DE-SC0019370.
