Scholes lab finds two chemical reactions coupled by quantum weirdness
Using the tools of chemistry–blue light, a room temperature environment, a reaction in solution–researchers in the Scholes Group record the very signature of quantum weirdness correlating a pair of chemical reactions in their latest research, published this week.
By studying a pair of chemical reactions that occur with equal likelihood in a large molecule–one reaction happening on the left side of the molecule, and the other, indistinguishable, happening on the right—the team discovered that these reactions occur in weird quantum lockstep.

Ben Xinzi Zhang, former graduate student in the Scholes Group and first author on the research paper.
Their research seeks to bridge the boundary between the laws of quantum and classical mechanics, revealing the missing connection between quantum and the classical, stepwise paradigm through which chemists typically perceive chemical syntheses.
For instance, the classical perspective would force a clear distinction in the pathway: i.e., the reaction will happen either on the left or the right side of the molecule. In the quantum world, however, scientists cannot distinguish left versus right, and therefore expect an evolution of a superposition of the two possible reactions.
The Scholes Group’s research, which puts experiment and theory on equal footing, yields a definitive “yes.” They present a pair of proton-transfer reactions that proceed in quantum lockstep until the state spontaneously collapses into a (classical) left or right product.
And although it takes only 2/100,000,000,000,000ths of a second, the period of time before the reaction collapses is long enough to be tracked and measured through ultrafast spectroscopy.
“Let’s say you can observe the reactants and products in a reaction,” said Greg Scholes, the William S. Tod Professor of Chemistry. “When we pair up two reactions, the chemist’s intuitive picture of a reaction is that 50% of the time the reaction will happen on the left and 50% on the right side of our pair. And that’s that. Which, of course, is true.
“But what we suggest is that if we zoom in on the earliest stages, the reaction is happening in a superposition state. During the initial phase of the reaction—which is about 20 femtoseconds of a total 60-femtosecond reaction—those protons are being transferred as a superposition, a weird quantum state. That’s the big advance of this work.
“It is remarkable to discover,” Scholes added, “a test bed system for quantum evolution at this scale of complexity in a real chemical reaction. There was not one, previously. And now there is.”
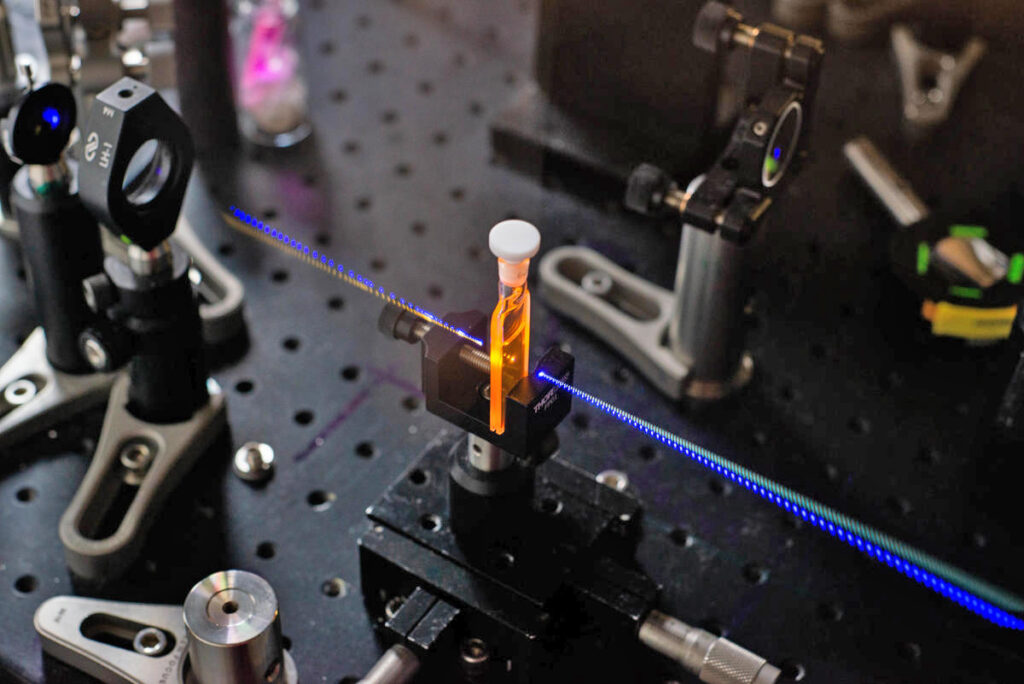
The group’s paper, “Interference of nuclear wavepackets in a pair of proton transfer reactions,” was published this week in the Proceedings of the National Academy of Sciences (PNAS). It fulfills a five-year, $1M project funded by the W.M. Keck Foundation.
In the investigation, chemists replaced one of the two protons in the reaction with a deuteron, so the particles would be distinguishable as researchers sought to track their movement. This enabled the team to turn the quantum mechanical interference between pathways from reactant to product on and off, which revealed a signature for the involvement of a superposition state in the reaction.
“In chemistry, it is crucial to understand where a reaction happens in a molecule. Here, we’re presenting a molecule in which you cannot be sure. That’s a serious intellectual challenge,” said Ben Xinzi Zhang, a former graduate student in the Scholes Group and first author on the paper.
“But we’re also saying, hold on, it’s okay, because we can use quantum mechanics to explain this. Even though it is not intuitive, it is understandable. So, in this case, when we make the system a tad bit heavier with the introduction of a deuteron, the reaction doesn’t proportionally slow down. It slows down a lot compared to the expectation of a statistical average.
“That slow-down,” Zhang said, “can only be explained with a core ingredient of the quantum theory: wavelike interference phenomena. That is the discovery here. “I think quantum mechanics is not as crazy as it sounds, it’s just a different way of seeing things. It’s just that the intuition is harder to grasp,” said Zhang.
“What we found here is extremely rare. We had to have a lot of stars align in order to see this phenomenon. But it is possible to see it. It’s actually a pretty remarkable project, the way it worked out.”
For the full PNAS paper, click here.
The W. M. Keck Foundation was established in 1954 in Los Angeles by William Myron Keck, founder of The Superior Oil Company. One of the nation’s largest philanthropic organizations, the W. M. Keck Foundation supports outstanding science, engineering and medical research. The Foundation also supports undergraduate education and maintains a program within Southern California to support arts and culture, education, health and community service projects.
The authors of “Interference of nuclear wavepackets in a pair of proton transfer reactions” include: Ben Xinzi Zhang, Kyra N. Schwarz, Luhao Zhang, Francesca Fassioli, Bo Fu, Lucas O. Nguyen, Robert R. Knowles, and Gregory Scholes. The authors acknowledge the W. M. Keck Foundation for funding this project in its entirety.
