Schoop Delivers on Scalable 2D Materials Through Chemical Exfoliation
The Schoop Lab announces a simple chemical exfoliation method that produces a high-quality, aqueous, superconducting nanosheet ink – the first to be found stable in ambient conditions and to remain so for at least 30 days without any protective agents.
The monolayer sheets—which were synthesized in a scalable, two-step process from 2D topological insulator candidate 1T’-WS2 —are dispersed in a cheap, non-toxic and abundant ink solvent. Able to be cast on both hard and flexible substrates, the ink can be processed for potential use in printable superconducting electronics, quantum computing, integrated circuits, and flexible devices.
Five and a half years in the making, the lab’s exfoliation method, or rather the ambition to see it through, was part of Associate Professor of Chemistry Leslie Schoop’s proposal when she was hired at Princeton Chemistry. There was considerable skepticism about whether she could pull it off.
Today, her proof-of-concept paper appears in Science Advances: “Synthesis of an aqueous, air-stable, superconducting 1T’-WS2 monolayer-ink.”
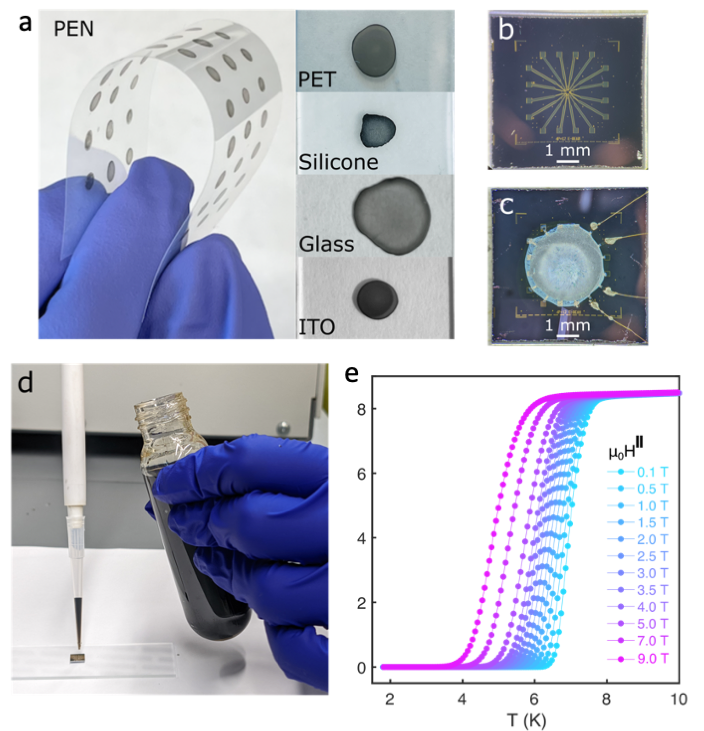
Graphics: (a) Printed films of the superconducting 1T'-WS2 ink on various substrates; (b,c) films printed on a pre-patterned wafer for electronic measurements; (d) a bottle of the ink and the process of printing through a nozzle; and (e) measured electronic resistivity under different applied fields showing that the film is superconducting.
“This is why I love this work so much,” said Schoop. “When I started this job, I said, ‘I’m going to make large quantities of 2D quantum materials through chemical exfoliation.’ People said to me, ‘You cannot do this because you will always have too many defects.’ I didn’t want to give up on it. It took us more than five years. I was lucky to have such a dedicated student as Xiaoyu Song to make my idea work.
“Now, we have shown that these nanosheets are very low in defects. We have these beautiful images of the atomic order in these systems: you can look at the electron microscope images and see the individual atoms, and they’re in a perfect, crystalline lattice. And the fact that the film superconducts is proof that they’re of high quality.
“As to the amount of sheets and ink you can produce through this method, it also has a pretty high yield,” added Schoop. “The yield is about 25%, which sounds low for organic synthesis but for exfoliation is ridiculously high.”
When the lab began their work on this problem, they were seeking a way to reduce bulk form WS2 to monolayers, but not through the traditional method of using scotch tape to exfoliate. That method is effective but not scalable.
This lab’s solution to this challenge came clear when researchers interposed potassium ions within the material layers just as if they were building a layer cake, in the words of the paper’s lead author Xiaoyu Song, a recent graduate of the Schoop lab. Researchers believed that this process seemed to “glue” the stacking layers together more firmly than stacked layers without the “filling,” Song said.
Xiaoyu Song, former graduate student in the Schoop Lab and lead author on the research paper.
From there, researchers were able to obtain clean, high-quality sheets through sonication, essentially shaking the material layers, suspended in water, apart.
Ratnadwip Singha, a postdoc in the lab and second author on the research paper, explained. “So what happens is, you put the bulk material in a water and acid solution. You get hydrogen gas from acid and it actually peels apart the layers from each other, it gently exfoliates this material and makes individual layers. Then, you get the suspension of these monolayers in water.
“If you have some alkali metals in between the layers the distance increases between them. So if you can now somehow remove the metal atoms from between those two layers, you still have a large distance. It’s like taking out the spacer. If you have a larger distance, it’s easier to pull the layers apart during sonication.
“The main thing is that we don’t need any protective molecules. And then also we show that it’s very stable. It’s very robust. We exposed the whole device, the ink, in the air for one month and it didn’t change anything at all.”
The patent-pending ink is metallic at room temperature and superconducting below 7.3K. While many materials have long been known to superconduct near absolute zero, the ability to do this at seven degrees above absolute zero is a notable development.

Ratnadwip Singha, a postdoc in the Schoop Lab and second author on the research paper.
Through four years of Song’s Ph.D. working primarily on this project, she was interested in the materials synthesis that would provide a foundation for the exfoliation method.
“To better design chemical exfoliation methods that could make materials of quality that met our standards, I spent a year and half studying the mechanism to understand the changes of structure and property relationships on the CrSe2 system,” said Song. “So, when it came to 1T’-WS2 , it was pretty fast with the knowledge that we acquired from the previous studies. It took only three months to do the exploratory experiments to understand the chemistry of this system that led to the design of this simple exfoliation method.
“I was super excited about the results. It took years to realize that ‘filling layers’ are actually the driving force that exfoliates these ‘layered cake’ materials. But then, when Ratnadwip and I stood in front of our Physical Properties Measurement System (PPMS) watching the resistance of our printed film made by this ink drop to zero, that excitement is hard to describe with language.”
Read the full paper in Science Advances.
This research was supported by DOD ONR (N00014-21-1-2733), NSF-MRSEC program (DMR-2011750), the Gordon and Betty Moore Foundation (GBMF9064). The authors acknowledge the use of Princeton’s Imaging and Analysis Center, which is partially supported by the Princeton Center for Complex Materials, a National Science Foundation (NSF)-MRSEC program (DMR-2011750).
