Schoop Lab Finds Facile Route to Flat Bands
The Schoop Lab has found a method to access flat bands in kagome lattice materials with a process nearly as simple as flicking on a laboratory light, but with broad ramifications for tapping the renewed interest in these systems.
Optimizing kagome materials and their properties for further investigation could provide avenues to emergent phenomena like superconductivity or magnetism.
Using so-called soft chemical processing methods that are not often explored in the quantum materials community, researchers oxidized Cs2Ni3S4, a compound that hadn’t been worked with since the late 1970s, to CsNi3S4 by plunging the material into an acid bath. That process knocked off a cesium atom, creating an unreported kagome compound.
“With soft chemical processing, what we’re aiming to do is take those solid-state-made crystals and just gently optimize them to be able to get at those properties that we really want,” said Grace Villalpando, lead author on the paper; she received her doctoral degree in May. “Maybe we want to just take a single atom out of this structure. Maybe we need to slightly oxidize it. But what we don’t want to do is lose the integrity of the material itself.
“This leads to really fascinating properties that are of interest to the physics community. When these bands are really flat, they can lead to strongly correlated systems where the electrons are not behaving as we would expect – they’re strongly interacting with each other.”
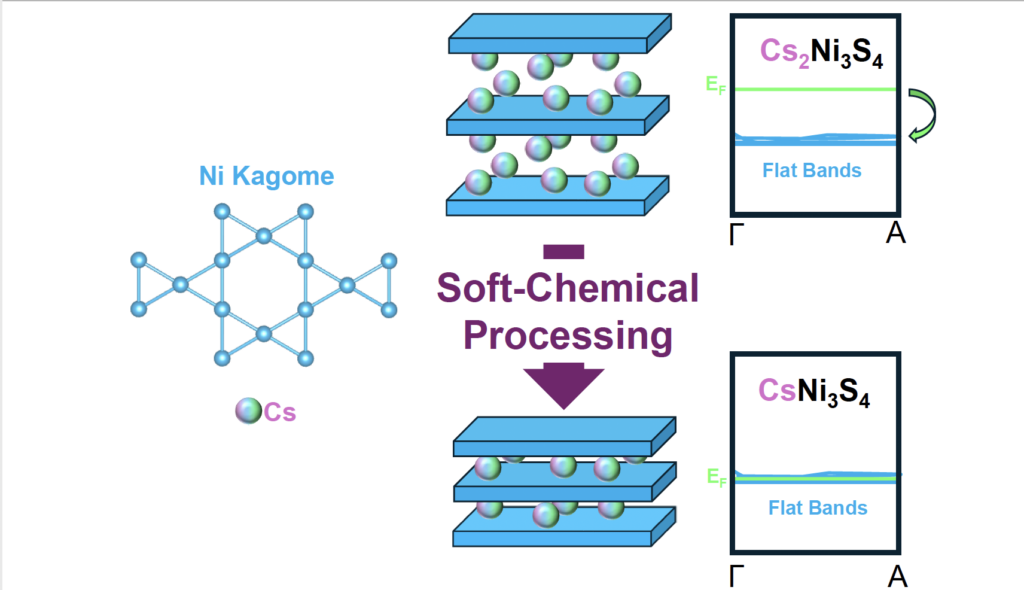
Nickel kagome containing compound Cs2Ni3S4 becoming CsNi3S4 through soft-chemical processing, resulting in the Fermi level moving to the flat-band region.
Professor of Chemistry Leslie Schoop underscored the significance of highly correlated systems. “When you have all these electrons squeezed together, we say that there are correlations because they know about each other and they’re all squeezed into this tiny energy window,” said Schoop. “And this is when you can get phenomena like superconductivity or other really cool phenomena that appear in solids and only in solids … but they appear when the electrons have these strong correlations.
“That’s why we look for these flat bands.”
Typically, flat bands in certain materials are not easy to access because they are at very low energies, far below the potential at which electronics are relevant in devices. The Schoop Lab’s aim was to find a chemical way to access them. Their paper, Accessing Bands with Extended Quantum Metric in Kagome Cs2Ni3S4 Through Soft Chemical Processing, was published in Science Advances this week.
Starting with a previous Nature paper
One of the Schoop Lab’s starting points was a 2022 paper published in Nature by Princeton Professor of Physics Bogdan Bernevig, who introduced a catalogue of flat-band stoichiometric materials. The flat bands used in this investigation, made from molecular orbitals of nickel and sulfur, have unique properties known as an extended quantum metric, which is typically used to determine whether flat bands can result in correlated physics.
The collaboration lays out a simple route to get to predicted flat bands with an extended quantum metric below the Fermi level.
“By tuning the Fermi level through chemical processing, we see significant changes in the material’s behavior, including signs of a possible correlated insulating state,” said Bernevig. “This research shows how chemical methods can effectively control flat bands in real materials. It highlights the role of quantum geometry in identifying non-atomic flat bands that may lead to correlated phenomena like superconductivity and magnetism.”

Grace Villalpando, lead author on the paper. She received her doctoral degree from the Department of Chemistry in May.
Normally, when trying to make these highly crystalline materials, researchers combine their starting materials and heat them to very high temperatures of 800 degrees Celsius or more. But not all materials are accessible this way, and Villalpando did not want to “destroy” the kagome in the parent material. So, she decided to start with something simple by using methods that optimize gently.
“I had no idea it was going to be so simple, but I could see right away that it worked. I put it into the dilute acid, which in turn removed a single cesium and then changed the oxidation state of my nickel atom. That moved my Fermi level to flat bands while still keeping the kagome structure intact.
“It does something a little weird in that it only oxidizes one out of the three nickels when it kicks out one of the cesiums, but for some reason it makes this unreported material incredibly air stable. Which is another selling point for this method.”
Added Schoop: “What we discovered is that, based on all the information we could gather, that theory predicts the ground state to be metallic. But we measure it to be insulating. And that usually means there’s an interesting reason for it, like that it’s correlated.
“We couldn’t solve every mystery that we uncovered for this compound. So now, I hope the physics community will pick up the investigation from here.”
