Simulations reveal dynamics of electrons at the surface of popular photocatalyst TiO2
Theoretical chemists in the Selloni lab at Princeton University have offered a detailed picture of the dynamics of excess electrons near anatase titanium dioxide (TiO2) surfaces. Understanding this behavior is crucial for improving the efficiency of this material in valuable solar cell and photocatalytic applications, such as water splitting, which turns water into oxygen and hydrogen, a clean alternative energy fuel.
“Titanium dioxide is a very cheap and environmentally friendly material and a widely used photocatalyst, but rather inefficient. If you could improve its efficiency, for instance for water splitting, it would be quite a response to our energy problems,” said Sencer Selcuk, postdoctoral researcher in the Selloni lab and lead author on the study.
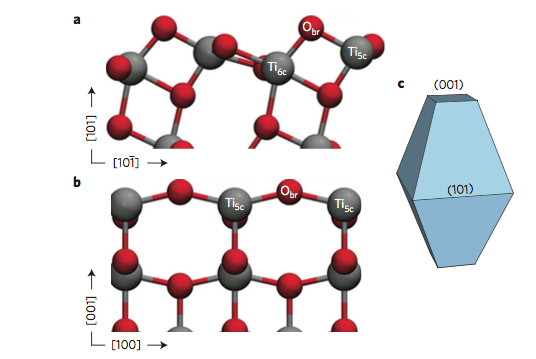
Published in the journal Nature Materials, these simulations revealed that the two common surfaces of anatase titanium dioxide, (001) and (101), behave oppositely in their accommodation of electrons. Though experimental observations of this behavior have been previously reported, these simulations provide the first fundamental explanation of the electrons’ behavior.
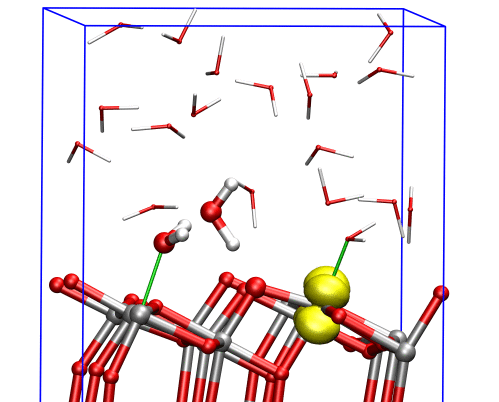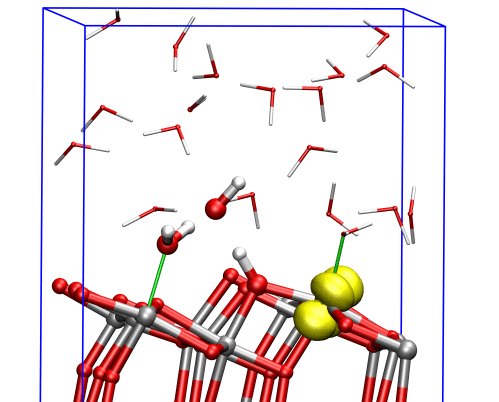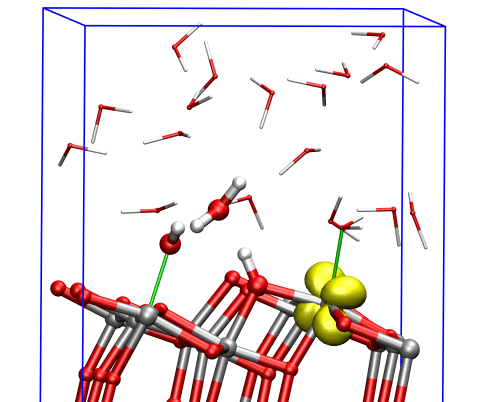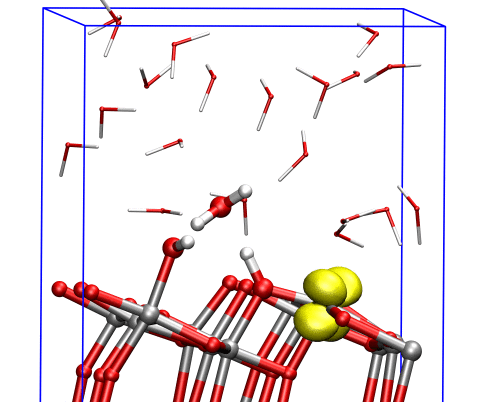
The researchers found that at the (001) surface the electron avoided the aqueous interface and remained in the interior of the material, moving between titanium dioxide’s inner bulk layers. But at the (101) surface, the electron could easily reach the interface, where it triggered dissociation of a water molecule nearby, and the two species formed a complex that was stable over the timescale of the simulations.
This computational approach allowed the researchers to study the electrons’ behavior near the TiO2 aqueous interface, providing a link between experimental measurements in ultrahigh vacuum and real applications in aqueous environments.
“Titanium dioxide has been studied for more than four decades,” Selcuk said, “yet, it its popularity among the scientific community has not waned.”
Read the full article here:
Selcuk, S.; Selloni, A. “Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces.” Published online ahead of print on June 20, 2016.
This work was supported by DoE-BES, Division of Chemical Sciences, Geosciences 91 and Biosciences (DE-FG02-12ER16286) and by the 92 National Energy Research Scientific Computing Center (DoE Contract No. 93 DE-AC02-05CH11231).
