Stache Opens New Depolymerization Path with LED Light and Quantum Dots
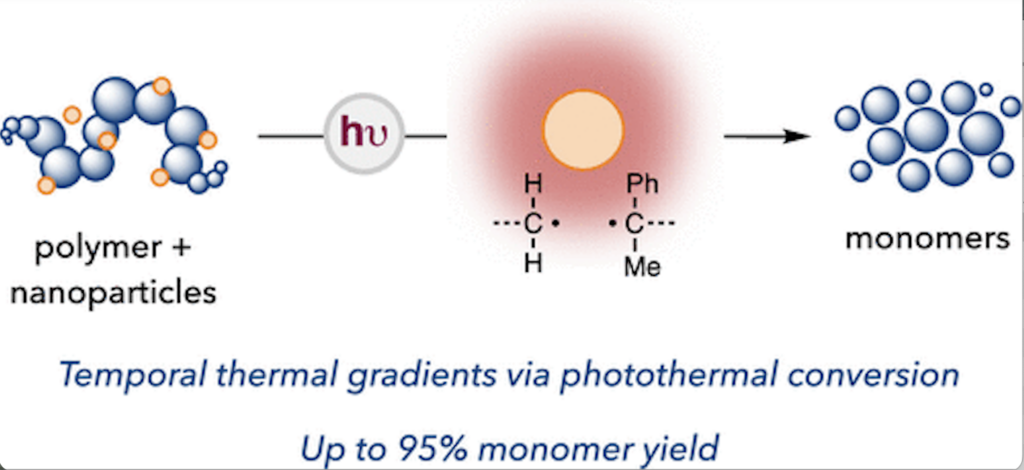
Using photothermal energy to advance chemical recycling, the Stache Lab announces a depolymerization method that relies on heat generated by nanoparticles to affect a broad level of spatial and temporal control.
In research published this week, the team activated carbon quantum dots, which they introduced into bulk polymers, with LED light. Upon irradiation, the nanoparticles generated localized thermal gradients capable of depolymerizing certain commercial plastics – in which only the portion of the material proximate to the nanoparticles is unzipped.

From left: second-year graduate student Clotilde Tagnon, Assistant Professor of Chemistry Erin Stache, and lead author on the paper Liat Kugelmass.
In addition, the side reactions and byproducts typically associated with pyrolysis—the industry standard for scalable chemical recycling—do not occur to the same degree with the Stache strategy.
The lab’s research marks the first time chemists demonstrate the utility of photothermal conversion using carbon quantum dots for chemical recycling.
It also highlights an exciting new direction in sustainable chemistry for one of Princeton Chemistry’s newest assistant professors, Erin Stache.
“This work came out of discussions about photocatalytic non-radiative decay pathways that could generate heat as a stimulus,” said Stache. “We were looking for a technique that would give us selective monomer generation with spatial and temporal control. The advantage of spatial control is that in a mixture of two polymers where only one polymer has this nanomaterial embedded, only the material with the quantum dots is going to be depolymerized by shining light on it. That’s the spatial side.
“Then there’s the temporal selectivity,” she added. “You can imagine that all these polymers depolymerize at different temperatures. So you can tune the intensity or the wavelength of light to access those different thermal gradients. Then you can do a selective, iterative depolymerization. You irradiate one polymer, you take off the monomer. You irradiate the next polymer, you take off that monomer. And so on.”
The lab’s paper, Photothermal Mediated Chemical Recycling to Monomers via Carbon Quantum Dots, was published this week in the Journal of the American Chemical Society (JACS) by Stache, lead author Liat Kugelmass, and second-year graduate student Clotilde Tagnon.
“My interest in the value of the greater sustainability mission was crucial in keeping my motivation throughout the three long years of this research,” said Kugelmass, who will defend her Ph.D. later this month. “While this work was polymer-focused, we envision broader utility of our discoveries to the chemistry community. We hope this will be the start of many photothermal projects to come out of the Stache group.”
How It Works
Plasmonic nanomaterials like gold and silver, for example, have already been proven to generate heat far in excess of temperatures needed for depolymerization. But the Stache Lab wanted to get away from a process that uses expensive, precious metals. So they came up with the idea of using carbon-based nanomaterials, instead.
Quantum dots have a very high level of fluorescence. But Stache wanted dots that give off heat, not light. So researchers in her lab preferentially synthesized low-fluorescence dots. The nanoparticles worked almost immediately.
“There are so many nanoparticles that can accept light as an energy source and then do things with it,” said Tagnon. “This is the first instance for depolymerization where we’re using things that take the light energy in and release it as heat instead of releasing it as equivalent chemical energy. That’s what is so special about this strategy.
“The field of carbon quantum dots is only about 20 years old,” they added. “For the first 15 years, researchers focused solely on fluorescence. And then for the last five years people were saying, oh, this heat release is pretty interesting. Let’s see what it can do. But no one had applied that concept to depolymerization. We looked for dots in the literature that were known to release heat so we could use the photothermal effect. This is something that feels like such a win for me.”
The nanoparticles are spun down from solution, dried, incorporated into the polymer matrix, and then hit with light. The light induces depolymerization.
One of the biggest challenges for the future of Stache’s process is scalability, or, bringing the method to the level of chemical recycling as practiced today in massive industrial reactors.
“The other intriguing question is, how should we incorporate these nanomaterials to induce the depolymerization?” said Stache. “We’re doing it now in a post-consumer waste protocol. Do we want that, or can we manufacture plastics with the nanomaterial embedded, and then just have to trigger depolymerization at the right time?
“That,” she said, “is a really exciting opportunity.”
This research was supported in part with funding from the National Science Foundation through MRI Award CHE-1531632 and the Cornell Center for Materials Research Facilities, supported by National Science Foundation Award DMR-1719875.
