YANG ADVANCES SINGLE-MOLECULE TRACKING WITH NOVEL IMAGING METHOD

The Yang Lab reports a novel imaging platform that employs a hardware-based “time-gating” module and two-photon excitation to track a single, luminous nanoparticle in real time in highly complex systems.
An exciting, intermediate step in the ongoing advancement of nanoprobe analysis and measurement, the platform allows the tracking of nanoparticles in three dimensions and at very high speeds, something that was previously challenging in real time owing to the overwhelming presence of background “noise.”
The technology is analogous to pinpointing light from a single star in an urban nightscape drenched in artificial light. It will equip scientists to investigate the dynamics of fast particles in their native systems, from biological cells to complex nanoscale materials.
The platform uses a gating system timed to a laser pulse that bars early, short-lifetime signals arriving from a nanoprobe – emitted by irrelevant organelles, cell walls, or intrinsic materials that are largely noise – from entering the imaging gate, but permitting later signals, or photons, to enter.
In this way, photons of interest can be precisely tracked throughout their lifetimes, spatially and temporally. The rate of tracking is estimated at 100 MHz, some 1,000 times faster than previous, imaging-based tracking methods.
“Using the star analogy, how long has it taken for the photon from that star to arrive?” said Joseph Beckwith, a postdoctoral researcher with the Yang Lab and a co-author on the paper. “The photon from the star takes a lot longer to arrive than all of this urban light pollution, which is already there. So just by knowing that arrived much sooner, we can ignore it. If it arrives much later, then that is the thing we’re interested in.
“The whole advance of this paper is looking at when the photons arrive at the detectors.”
The collaborative research, “Leveraging lifetime information to perform real-time 3D single-particle tracking in noisy environments,” was published in the Journal of Chemical Physics and made possible by work from the Department of Chemistry, University of Illinois at Chicago; and the Department of Biochemistry and Molecular Biology, Pennsylvania State University. Lead authors on the paper are Tian Zhao, a postdoctoral researcher in the lab, and Haw Yang, professor of chemistry.
“To understand chemical reactions in complex systems, we need to measure stuff in those reactions. The biological cells that make up our bodies and all living organisms, for example, have thousands and thousands of components acting together and acting separately. Trying to measure anything in that environment is just challenging in itself,” said M. Junaid Amin, a postdoctoral researcher with the Yang Lab and a co-author on the research.
“We want to understand how life happens, how chemistry happens. So, we need to understand complex systems like this one. Tian’s beautiful paper helps create some instrumentation in that direction.”
MADE IN THE YANG LAB
Using the lab’s in-depth experience with optics, researchers built the precise detection system needed for their experiments in the basement of Frick Laboratory.
Essentially, their method relies on a series of detectors arrayed so as to pick up the slightest change of course in the particle, or probe. The system adjusts for that change in real-time so that the probe’s position can be continuously plotted. Researchers set the gating at a fixed position. When a laser pulse hits the sample of interest, the clock starts. The gate will only accept and send on to the detectors photons emitted after a certain time window.
The rejection of background light is improved by using two photons to excite the emitting particles, instead of the usual one.
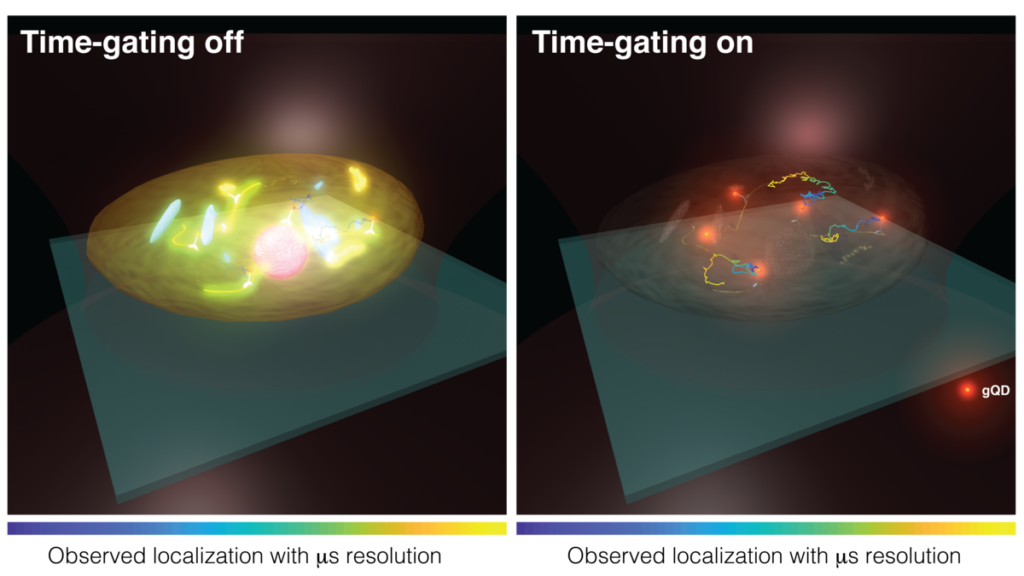
Time-gating can efficiently reduce the short-lifetime signals inside of the biological structures and only send out the longer-lifetime signals that correlate with the units of interest.
“When you shine the laser, many things can illuminate, many things can scatter,” said Zhao. “But if you are using two-photon excitation – that means, you give the particle two photons that both have lower energy – and they combine, and they excite the sample, then the sample releases a higher-energy photon. We need the pulsed laser that can provide very high laser power in each single pulse to achieve this.
“This is actually a very important feature that benefits biological imaging because only the region of the sample at the focus can receive such high-level power from the laser to emit photons. Any out-of-focal areas cannot receive such high powers to generate two-photon induced emissions.”
The research builds on core technology introduced by Yang in 2006 while he was at the University of California, Berkeley: the first application of 3D single-particle tracking spectroscopy. Several essential advances have been made along the way. This research marks the latest.
The time-gated tracking capacity should in future be able to be coupled with other imaging and control methods to broaden the scope of experimentally accessible systems.
Read the full Journal of Chemical Physics paper.
The research was also highlighted as an American Institute of Physics (AIP) “scilight,” here: https://aip.scitation.org/doi/10.1063/10.0006749.
This paper was authored by Tian Zhao, Joseph Beckwith, M. Junaid Amin, Marcell Pálmai, Preston Snee, Ming Tien, and Haw Yang. Funding for this research was provided by the Department of Energy, DE-SC0019364; and the Eric and Wendy Schmidt Transformative Technology Fund.
