Hyster Lab uses photoenzymatic catalysis to make non-canonical amino acids
Uncovering new catalytic functions for enzymes once thought to be limited in functionality, the Hyster Lab reports a streamlined method to prepare non-canonical tertiary amino acids, essential components of drugs and agrochemicals.
In their research, the lab exploits pyridoxal (PLP)-dependent enzyme threonine aldolases and their interaction with pyridinium salts and a photoredox catalyst. The enzyme localizes radical formation to an active protein site and does away with the cumbersome steps typically needed to protect and deprotect reactivity within the molecule.
Their method allows for “nearly perfect” enantioselectivity and adds a new enzyme to the photoenzymatic catalysis repertoire.
The lab’s paper, Synergistic Photoenzymatic Catalysis Enables Synthesis of a-Tertiary Amino Acids Using Threonine Aldolases, was published last month in the Journal of the American Chemical Society (JACS).

Over the past 10 years, the pharmaceutical industry has expanded its interest from small molecule therapeutics to therapeutics that look more like proteins or peptides. In order to modify the functionality of these molecules, chemists need to include functional groups that aren’t found in the 20 canonical amino acids. Thus, the need for methods to make and rapidly access them.
“When you first start studying enzymes, you’re told that they can only do very specific things, that they’re restricted to do only what they do in nature. But our group demonstrated a few years ago that you can use photoredox catalysts with enzymes to unlock new transformations,” said Professor of Chemistry Todd Hyster.
“What we found is that when given new substrates, many enzymes have a latent photoenzymatic function that nature just doesn’t take advantage of. So here, we took that general strategy and brought it to a different class of proteins to be able to get to molecules that have high medicinal and therapeutic values.”
The paper also provides one of the first mechanistic studies to explain how the enzyme controls the radical formation and the selectivity.
“We are doing fundamental research. You can’t just develop and run a reaction without any mechanistic discussion,” said first author and Hyster Lab postdoc Yao Ouyang. “You have to educate people in the field to provide information on how we achieve this, how this happened, so that people understand your chemistry.
“There are a lot of papers that came out on this subject already, but the mechanistic details are unclear. In this project, we make them clear.”
Tertiaries are the tougher challenge
Methods to generate secondary amino acids have existed for years and are commonly used in photoenzymatic catalysis. But the Hyster Lab took a more challenging path with this research. It made tertiary amino acids, which in many cases are more desirable because they lead to improved functionality in medicinal applications.
“Tertiary amino acids are difficult to make without protection steps due to the incompatibility of the amine and carboxylic acid with most reaction conditions to make them,” said Claire Page, a paper author and graduate student in the Hyster Lab. “They are desirable in pharma because they can add rigidity to peptide structures and improve metabolic stability.”
Researchers believe their method is enabled by a unique ternary interaction, fueled first by an interaction between the enzyme and the photocatalyst Rose Bengal (RB), and then between RB and the radical precursor pyridinium salt.
“Here, the external photocatalyst RB acted as a bridge that mediated a three-component interaction between enzyme, RB, and pyridium salt,” Ouyang explained. “This unique interaction provides strong evidence of how the radical generated by the exogenous catalyst can participate in the highly selective C-C bond formation in photoenzymatic catalysis.”
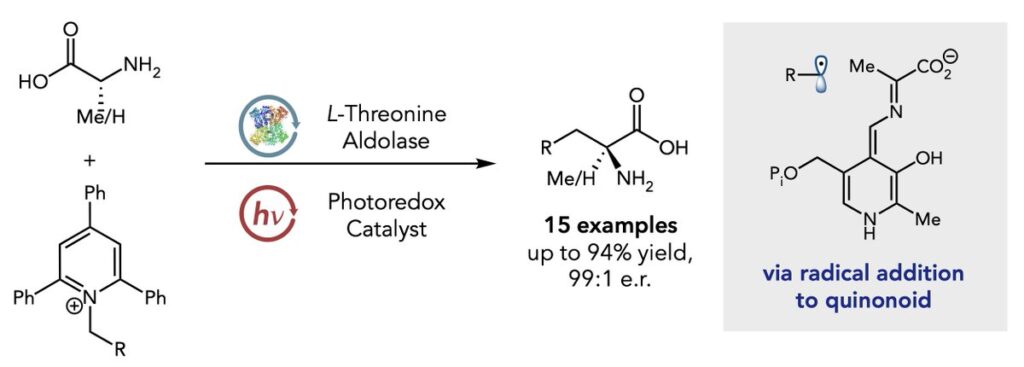
Hyster also explained the reduction in steps traditionally needed for protection and deprotection, saying his lab took its inspiration from nature. Nature doesn’t use protecting groups. Instead, it binds the amino acid and protects the acid through interactions with the protein.
“With the PLP co-factor, you’re able to protect the amine and activate it,” said Hyster. “The protein is essentially doing those protecting and activating steps so that we can just do the functionalization, and then it will release the product at the end in its unprotected form.
“What we realized is that there is an electropositive region of the protein that interacts with our negatively charged photocatalyst, and that’s what’s responsible, we think, for binding the photocatalyst to the protein. The most reactive radical precursor for us is positively charged, and so its positive charge allows it to interact strongly with a negatively charged photocatalyst.”
Added Ouyang: “This paper is a real contribution to the field. It paves the way for future research on how to design synergistic photocatalysis.”
Authors include: Yao Ouyang, Claire Page, Catherine Bilodeau, and Todd Hyster. Full paper here: https://pubs.acs.org/doi/10.1021/jacs.4c04661
This research was supported by the National Institutes of Health National Institute of General Medical Sciences (R21GM146042).
