Illuminating Chromatin: Muir and MacMillan Labs Light the Way
Studying the microbiology of any entity, be it a molecule or a dolphin, ideally means putting a spotlight as close to the source material as possible. That can be especially challenging when you’re investigating the Rube Goldberg environment of a cell’s nucleus.
But in research published this week in Nature, the Muir Lab and the MacMillan Lab used two of Princeton Chemistry’s marquee technologies to shine a light right where they wanted it.
In the process, they discovered critical, unexpected changes in interactions surrounding a DNA-protein complex called chromatin—essentially, an architecture that allows for the compaction of DNA—in the presence of genetic mutations widely associated with cancer.

Authors on the research, clockwise from top left: Thomas Muir, the Van Zandt Williams Jr. Class of 1965 Professor; David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry; Antony Burton, formerly of the Muir Lab and co-lead author on the paper; and Ciaran Seath, formerly of the MacMillan Lab and co-lead author on the paper.
Interactions between biomolecules control every biological function. Mapping their activity leads to a deeper understanding of cell fate. So researchers paired the strengths of µMap, the MacMillan Lab’s proximity-labeling system introduced three years ago, with in nucleo protein trans-splicing, a protein engineering technology introduced in 2016 and optimized since by the Muir Lab. Through these technologies, they were able to tether an iridium photocatalyst to a protein of interest to study these minute interactions and how they change in the presence of mutations—all without impacting the complex microenvironment within the cell nucleus.
The photocatalyst highlighted a radius of focus just one-nucleosome wide, allowing researchers to peer into this microenvironment with unprecedented specificity.
“So many things in biology and disease come down to how chromatin moves and changes,” said Ciaran Seath, formerly of the MacMillan Lab and co-lead author on the paper with Antony Burton, formerly of the Muir Lab. “Viruses, aging, cancers, all the things we looked at—change how chromatin can move and react. We figured, if you could watch that, you could learn about all of these different problems in a modular way.
“Sometimes you can’t see or predict all the other things that are likely to happen in the machine of the nucleus. Now that we have paired these tools, we can measure these unforeseen consequences. It’s like touching a point on a spider’s web,” he said. “You can see the whole thing move.”
Burton added: “What the synergy of these technologies gets you, in a minimally perturbative way, is the ability to install a small catalyst onto a protein of interest and then map what’s nearby. We were able to illuminate protein interactions and complex, downstream effects at a level of detail that is very hard to do with other methods.
“Most importantly, we can chart how these change as a function of mutation or drug treatment, presenting opportunities for academic and industrial application.”
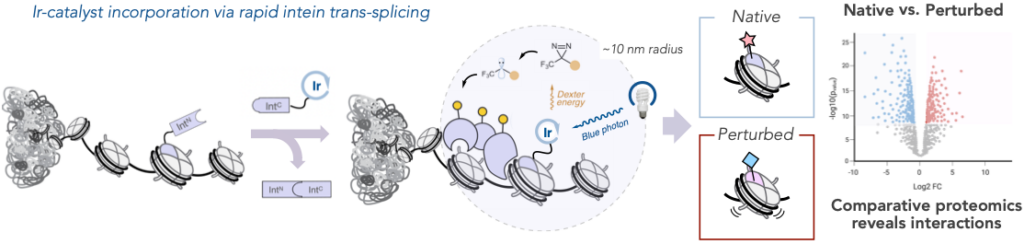
Graphic showing the strategy for nuclear photoproximity labeling; and comparative chemoproteomic analysis reveals how a given perturbation (e.g. a mutation or drug treatment) affects local interactomes, providing insights into the mechanistic basis for disease or therapy.
The research, Tracking chromatin state changes using nanoscale photo-proximity labeling, was authored by Seath, now an assistant professor of chemistry at the Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology in Jupiter, Florida; Burton, now a senior scientist in chemical biology at AstraZeneca in Boston, Massachusetts; and P.I.s Thomas Muir, the Van Zandt Williams Jr. Class of 1965 Professor, and David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry and co-recipient of the 2021 Nobel Prize in Chemistry.
Advancing the Field of Epigenetics
The research has important implications for epigenetics, the branch of biology that explores changes in gene expression. At the heart of epigenetics are histone proteins, which package DNA and restrict access to the genome, thereby playing a key role in regulating transcription.
Recently, mutations to these histone proteins have been discovered and linked to a wide range of cancers.
“One of the things we looked at was adding a mutation that occurs in a histone that’s linked to cancer,” said Muir. “What we wanted to know is, what happens when that mutation is present; what can no longer be recruited, what’s no longer in the neighborhood, what new things are brought in that shouldn’t normally be there?
“We were able to use these technologies to find all sort of things that get changed when we put this mutation on the chromatin, things related to gene regulation,” he added. “We were able to infer mechanistic insights that relate to how genes get misregulated with a mutation.”
Beginning their research three years ago, the team hypothesized that the mutation associated with cancer precipitates some sort of loss of function. Biomolecules find chromatin and bind with it to leave a transcriptional mark. Researchers believed that the mutation blocked some of that action, thus leading to cell dysfunction.
Their hypothesis was borne out with this research, which generated molecular detail on how a tiny change in the genome can lead to major effects.
“The whole point of µMap is to understand biology broadly defined in ways that you couldn’t beforehand because µMap gives you such incredibly precise information. This is an example of doing precisely that,” said MacMillan of his proximity-labeling technology.
“With this research, we saw that the biology is happening because of these mutations. It was impossible to see that before this research, so we were driving in the dark. It’s another piece in this whole wall, a brick of what µMap’s going to be able to do. It’s still just the beginning, but this is a true collaboration,” said MacMillan, “a true combination of technologies.”
Authors on the paper include: Ciaran P. Seath, Antony J. Burton, Xuemeng Sun, Gihoon Lee, Ralph E. Kleiner, David W. C. MacMillan, and Tom W. Muir. Read the full paper here.
This research was supported by funding from the National Institutes of Health National Institute of General Medical Sciences ((R35GM134897-01, R01- GM103558-03 and R37-GM086868) and the NIH National Cancer Institute (P01 CA196539).
