MacMillan Lab discovers two-step, “one pot” method to form fused, strained rings
Following is a summary of new research out from the MacMillan Group this week in Nature.
PAPER: “Couple-close construction of polycyclic rings from diradicals,” Nature.
AUTHORS: Alice Long, Christian Oswood, Christopher Kelly, Marian Bryan, and David MacMillan.
WHAT IT IS: In unprecedented work driven by the lab’s extensive knowledge of reagent-controlled radical generation, the MacMillan Group utilizes underexplored retrosynthesis for the convergent formation of a new class of strained bicyclic, fused, and spirocyclic compounds through two sequential radical-coupling reactions.
Their couple-close approach provides a new avenue for complex substrate diversification and substantial streamlining of a challenging synthetic sequence.

Lead author on the research and third-year graduate student Alice Long in the MacMillan Lab.
WHAT RESEARCHERS DID: Using bromo alcohols and diols as building blocks—readily available commercial feedstocks that also add to the appeal of this startling new method—researchers form complex ring systems with five, six, and even seven members. Traditional ring-formation methods for (semi)saturated rings, like Diels-Alder or transition metal catalysis, need multiple steps, sometimes as many as nine or 10. This work does it in a single vessel with two steps.
COMMENT FROM ALICE LONG, LEAD AUTHOR AND THIRD-YEAR GRAD STUDENT: “Once I was able to get the scope going and make the starting materials and then actually form very complex strained rings, I was really excited because I felt that this was going to be super impactful. I’ve worked in industry in the past, so I know how important it is to them. That’s the big impact of this work. They’ll be able to save a lot of time. You’re able to do it in one go versus nine or 10 steps.
“These building blocks that I’ve used for the diol part of the scope are really coming from amino acids, right? And amino acids as we know are just very cheap. If you can take something that’s a $1/gram and perform a one-step robust reaction to get to the diol… and you’re able to take enantiopure starting materials and form these rings, you’re able to retain some of the stereogenic centers when you form your ring. I think that concept is just so cool, that you’re able to use our chemistry and have it be stereo-retentive when you form these rings.”
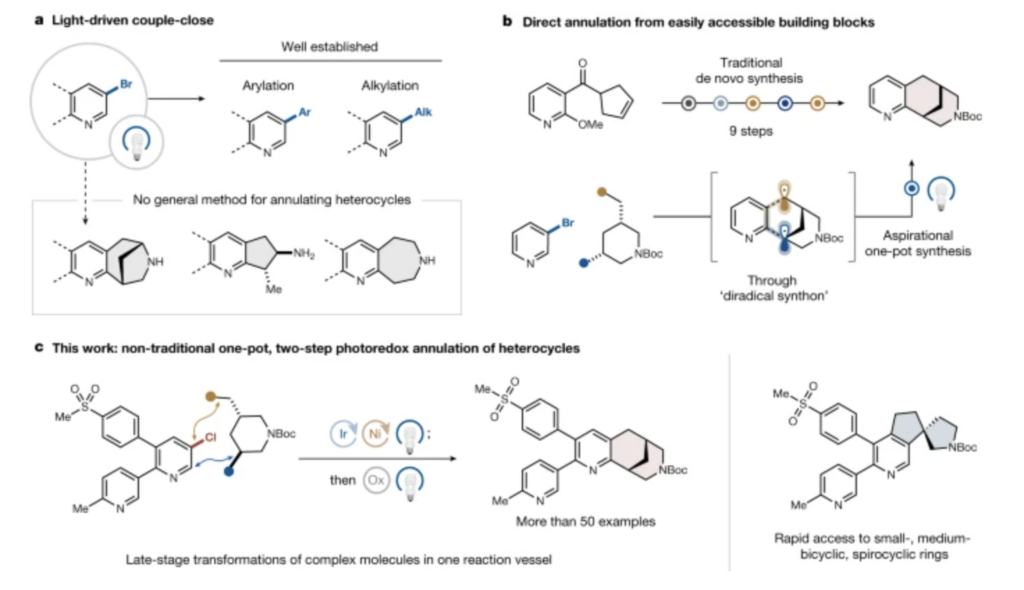
Traditional methods of constructing rings require lengthy synthesis. The success of ring construction relies on the successful merger between dual photoredox catalysis and nickel catalysis methods in a one-pot fashion.
COMMENT FROM P.I. DAVID MACMILLAN: “I love this research for two important reasons. One is because it is a new way to build up these molecules that include rings, these cyclic structures. We call it couple-close. So, you couple and then you close the ring. As far as we know, it’s a new strategy and new way of thinking about these things that allows you to build them much faster than you could before. This research builds off the advantages of photoredox catalysis, where you can generate these radicals that will couple and close, and you can do it all in one vessel.
“The second important thing is that it allows you to make these really, really strained molecules. That strain makes the bonds associated with it really strong at the sides for bizarre reasons. And for your body has a much harder time metabolizing it. Well, now the drug can last much longer in your body and do the work it has to over a much longer period of time.
“It’s going to be awesome. It’s a really cool new approach.”
FUNDING: Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS) under grant no. R35 GM134897-04; the Princeton Catalysis Initiative; Janssen R&D; and gifts from Merck, Bristol Myers Squibb, Genentech, GenMab and Pfizer.
