MacMillan Lab Scores Alcohol-Alcohol Cross-Coupling Method After Long Effort

In 2007, Senator Barack Obama was running for president, the final Harry Potter book was published, and David MacMillan, recently arrived at Princeton Chemistry, sat down with his lab members and challenged them to tackle the longstanding problem of coupling alcohols together.
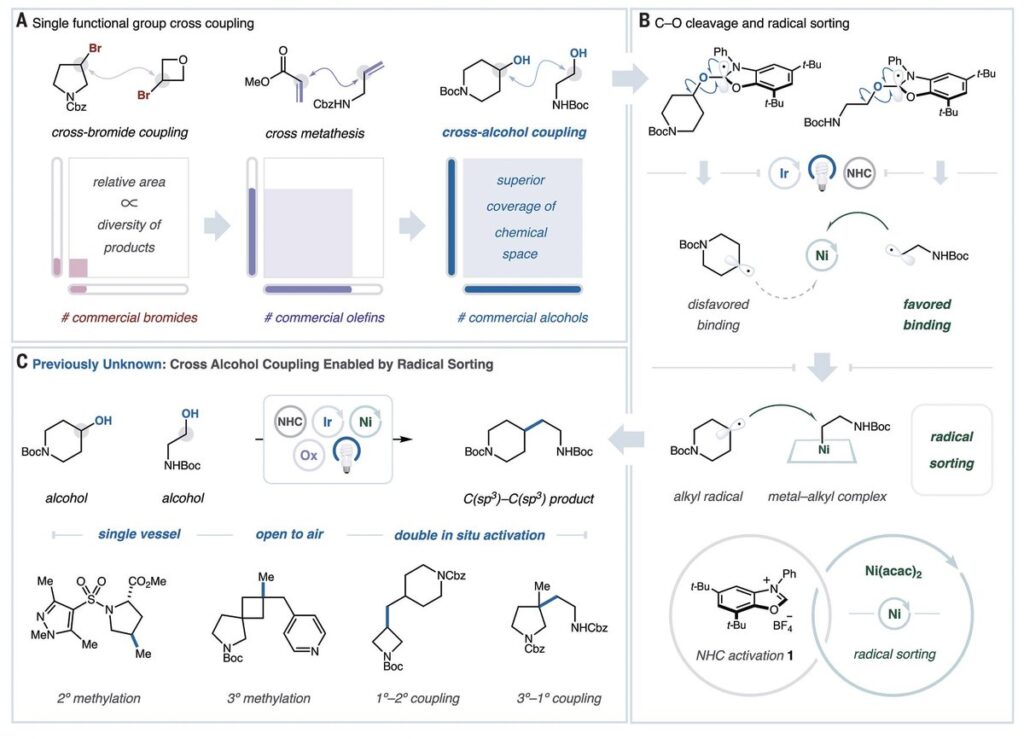
This month in Science, the MacMillan Lab publishes Alcohol-Alcohol Cross-Coupling Enabled by SH2 Radical Sorting, which details, at long last, their unprecedented success with a method that couples among the world’s most readily available molecules for new reactions and maximal chemical space.
MacMillan, the James S. McDonnell Distinguished University Professor in Chemistry, emphasizes that the research took 17 years to solve, which happens to be the kind of project he relishes most.
“I loved this problem because no one else was working on it. Those are the best. Some can be solved in a day. Some take 17 years. This one took 17 years, but it’s magical,” said MacMillan.
“If you think about medicines, what pharma is trying to do is make lots of different molecular shapes and interactions and then test them to see if they become a medicine. But that’s limited by the number of shapes and molecules you can build. Now all of the sudden, you can take what are among the most abundant feedstock molecules—alcohols—and couple them with each other. If you square that number, it’s enormous.
“It’s like having a million Lego pieces that you can suddenly couple with a million Lego pieces. It just gives you an enormous range of shapes and sizes and interactions that you could not readily join before. And it does it in one step. There’s nothing like this out there.”
Researchers said their process will enable the synthesis of new drugs, agrochemicals, fragrances, and other products, with immediate implications for the applied chemical sciences.
The team that pulled this off has built on years of contributions from graduate students and postdocs moving through the MacMillan group. They used two mechanisms advanced in the lab—photoredox-enabled deoxygenation of alcohols and radical sorting via bimolecular homolytic substitution (SH2)—to form new C-C bonds from abundant, bench-stable alcohols.
Graduate student Nicholas Intermaggio, a co-lead author on the paper, said the key realization was in bringing these two mechanisms together.
“The idea of taking these alcohols and cross-coupling them has been a sort of theoretical goal in our group for a very long time. On paper, it looks incredibly powerful. But there was just no way to do it,” he said.
“The way I see it now, our research stands on the shoulders of two previous, significant advances from our lab: the radical sorting SH2 Science paper in 2021, which shows you how to actually form the bond we want; and then the alcohol activation Nature paper, also in 2021, which shows you how to convert alcohols to radicals. So whereas before we were able to use one alcohol and some other functional groups that might not be quite as abundant or diverse, now we’re able to take two of the most abundant, most diverse and put them together for a much larger set of products.”
Simplicity and Accessibility
Co-lead author and first-year graduate student Ruizhe Chen said the simplicity of the technology is another mark of its achievement.
“It’s quite special that by not accessing a low-valent metal intermediate, our reaction can actually be run in ambient conditions,” said Chen. “So, you don’t have to take strict care regarding air or moisture for this transformation. We don’t need the glove box, we don’t need the nitrogen link, and we don’t need the Schlenk line. It is more easily reproducible, and the skill level required for chemists who will be operating this is also lower.
“That is another side that makes this chemistry powerful.”
The method: achieving alcohol-alcohol cross-coupling enabled by powerful SH2 radical sorting.
The alcohol-alcohol paper—the third published in either Science or Nature in the past four weeks—describes a process by which both alcohols react initially with an N-heterocyclic carbene (NHC) salt to form activated adducts. Photoredox catalysis cleaves the C–O bonds to produce reactive radicals, and then a nickel catalyst sorts those radicals to mediate formation of the C–C bond.
Selectivity inherent in the SH2 radical sorting platform enables the differentiation of radicals based on steric or electronic properties.
Co-lead author James Rossi-Ashton provided the original proof-of-concept for the cross-coupling about two years ago, shortly after the Dong paper was released. Rossi-Ashton surmised that the NHC/alcohol activation demonstrated in that paper would serve as an unbiased redox-active handle, eventually allowing the SH2 radical sorting to produce a successful deoxygenative cross coupling of two distinct alcohols. It was a clever assumption, and it worked.
“Of course, as with any process, it took some optimization to take the original 0.5% yield up to the yields you see in the publication today,” said Rossi-Ashton. “Really it is a tour de force and coming together and amalgamation of the hugely enabling synthetic methods that are currently coming out of the MacMillan group.”
Co-lead author Jiaxin Xie said the method is significant in the synthesis of complex molecules. “Usually we do retrosynthetic analysis with complex molecules, and sometimes we have to rely on established methods. And sometimes those methods can kind of limit our imagination about the potential starting materials,” he said. “But the wide availability of alcohols actually broadens our imagination.”
MacMillan said the technology constructs five distinct classes of C-C bonds from the most abundant, diverse alkyl fragments in a single, robust step: secondary and tertiary methylation, secondary and tertiay alkylation, and primary methylation.
Added Intermaggio: “There’s this term, dump-and-stir, to describe a reaction that is really easy to set up. This is not quite dump-and-stir, but it’s about as close to it as a photoredox reaction can get.
“I think that simplicity of the setup and the diversity of the products you can make is what really excites all of us.”
“Alcohol-Alcohol Cross-Coupling Enabled by SH2 Radical Sorting” was authored by Ruizhe Chen, Nicholas Intermaggio, Jiaxin Xie, James Rossi-Ashton, Colin Gould, Robert Martin, Jesús Alcázar, and David MacMillan. Funding for the research was provided by the National Institute of General Medical Sciences (NIGMS) (R35GM134897-04), the Princeton Catalysis Initiative, Janssen R&D, and gifts from Merck, Bristol Myers Squibb, GenMab, Genentech, and Pfizer.
